Search API
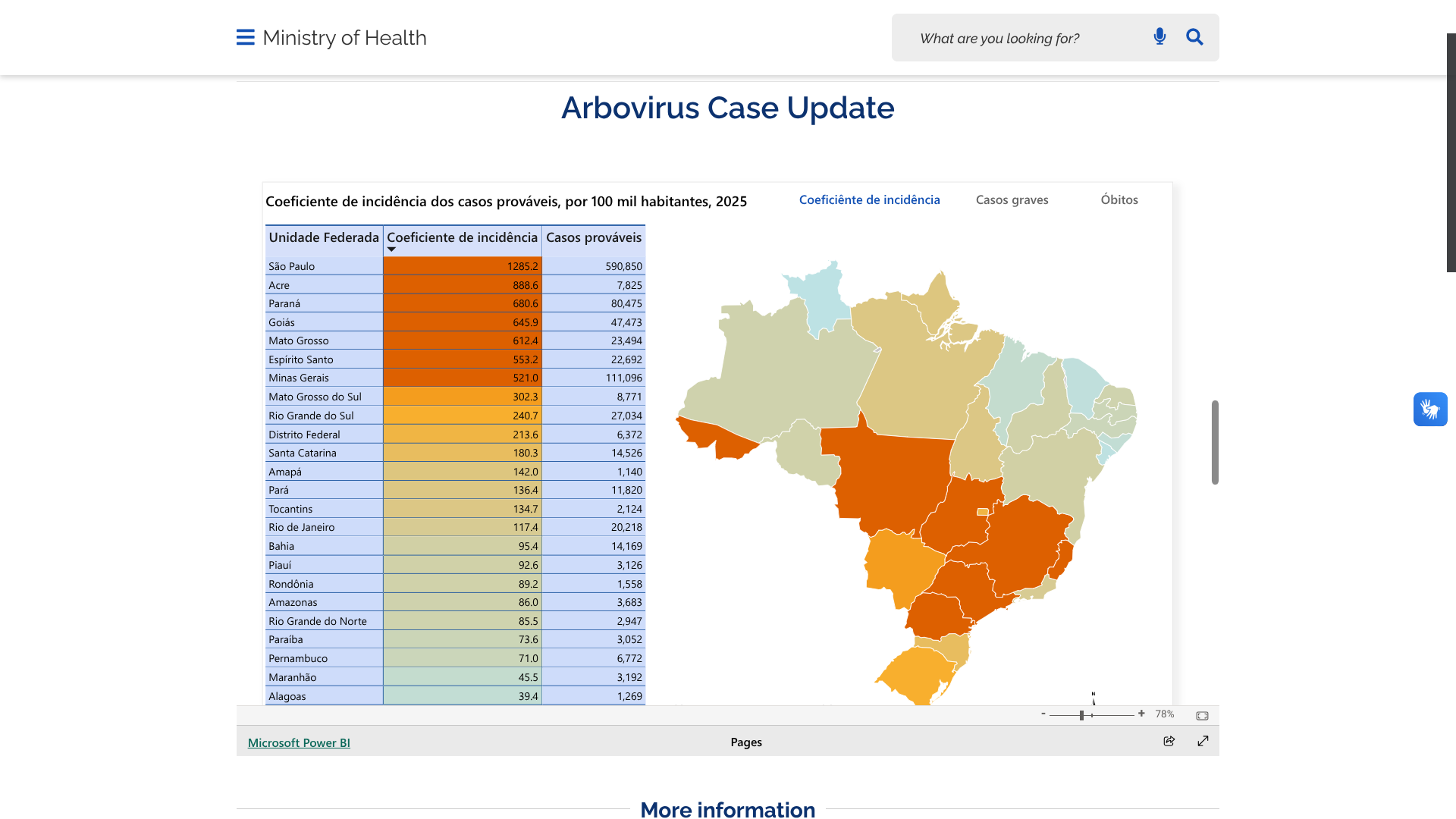
The Ministry of Health for the Federative Republic of Brazil recently confirmed a total of 1,019,033 dengue cases and 681 related fatalities this year.
As of April 12, 2025, São Paulo had reported the most dengue cases, at 590,850.
As of April 22, 2025, the U.S. Centers for Disease Control and Prevention (CDC) has identified more cases of dengue (1,481) than expected among U.S. travelers returning from countries with dengue outbreaks.
In 2025, 54 travel-associated and one locally acquired dengue cases were reported in Florida.
Of dengue's four types, DENV-3 is the most common serotype identified, accounting for 84%.
To alert travelers to Brazil and other areas, the CDC reissued a Global Travel Health Notice on April 15, 2025, regarding Dengue outbreaks in the Americas. Additionally, the CDC encourages healthcare providers to increase testing of patients with symptoms related to dengue.
Currently, dengue vaccines are unavailable in the U.S.
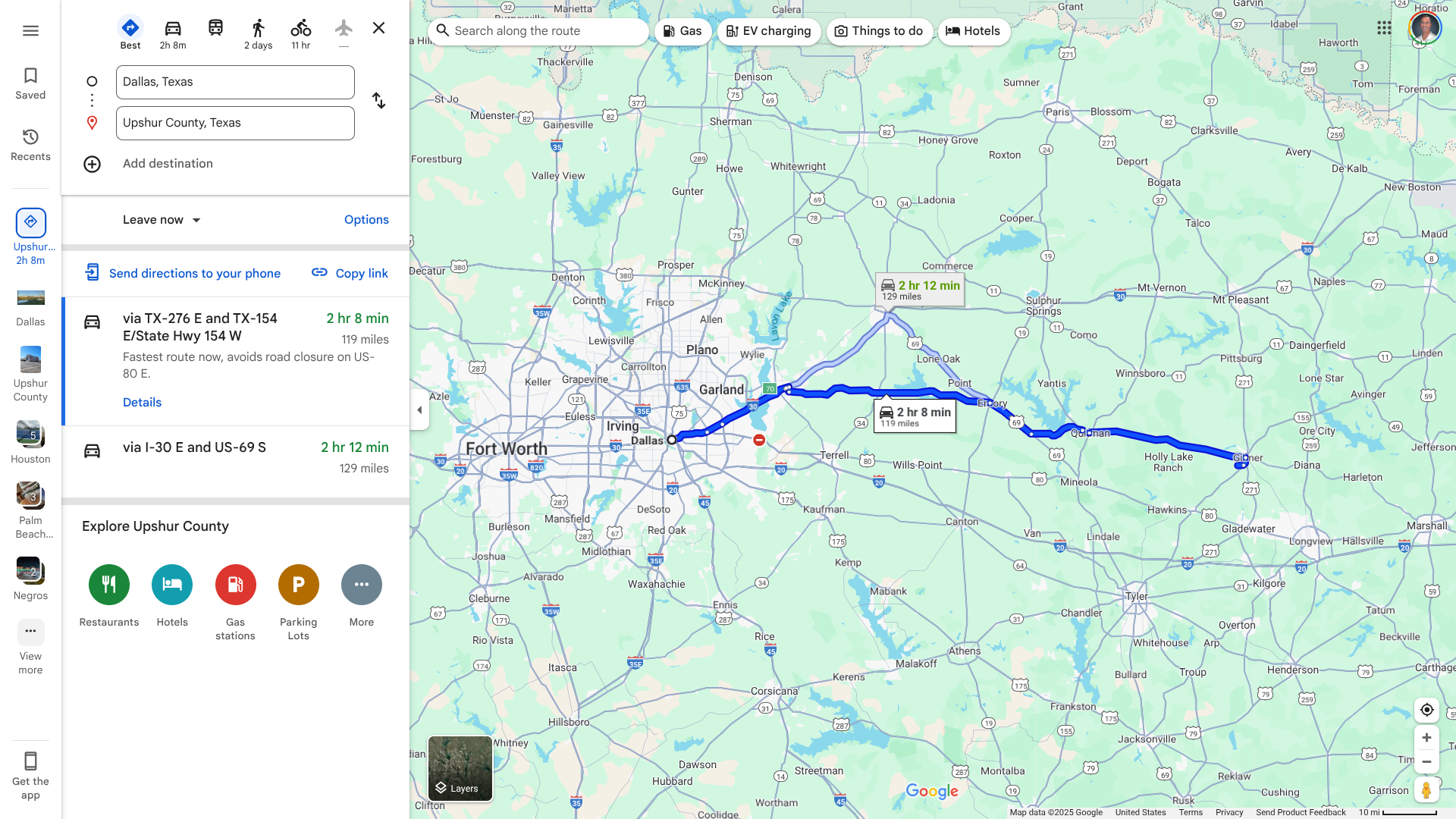
The epicenter of the measles outbreak in Texas appears to have moved about 120 miles east of Dallas to the sparsely populated Upshur County.
According to the Texas Department of State Health Services (DSHS) and a health department Facebook post, about 19 confirmed cases of measles were reported at a single location in Upshur County.
All cases involve individuals over the age of 17, with unknown vaccination status, who were linked to two individuals who visited Upshur County from outside the state. As of April 19, 2025, all affected persons were isolated from the public and are following all the appropriate guidelines.
These measles cases have not included any Upshur County residents, and there are no reports of measles in any of our Upshur County public schools, public buildings, county operations, or medical facilities.
DSHS stated that it is gathering additional information to determine the residency status of these cases and whether they are related to the West Texas measles outbreak in 2025, which has reached 597 cases.
Other counties in Texas have reported 10 measles cases in 2025.
Throughout the United States, 25 jurisdictions have reported about 800 measles cases this year.
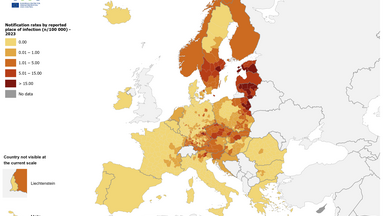
Recent maps released by the European Centre for Disease Prevention and Control (ECDC) indicate hotspots for tick-borne encephalitis (TBE) in Europe.
There are three distinct subtypes of tick-borne encephalitis virus: European, Far Eastern, and Siberian. They are found in different areas and cause disease of varying severity.
While the virus is already present in many European countries, as of April 15, 2025, the Central, Eastern, and Northern European regions have recently reported their first human cases of TBE or have noted an increase in infections.
In Europe, ticks become infected when they feed on small rodents that have the virus in their blood. People can become infected through the bites of infected ticks.
TBE is a vaccine-preventable disease that affects the central nervous system. Vaccination is an effective means of prevention.
In addition, taking personal protective measures, such as wearing long clothing, using tick repellent, avoiding tick-infested areas, and promptly removing any ticks from the skin, can significantly reduce the risk of infection.
According to the U.S. CDC, the TICOVAC tick-borne encephalitis vaccine is available in the United States and is approved for use in persons aged 1 year and older.
As of April 21, 2025, the CDC recommends that TICOVAC be considered for some travelers visiting high-risk areas.
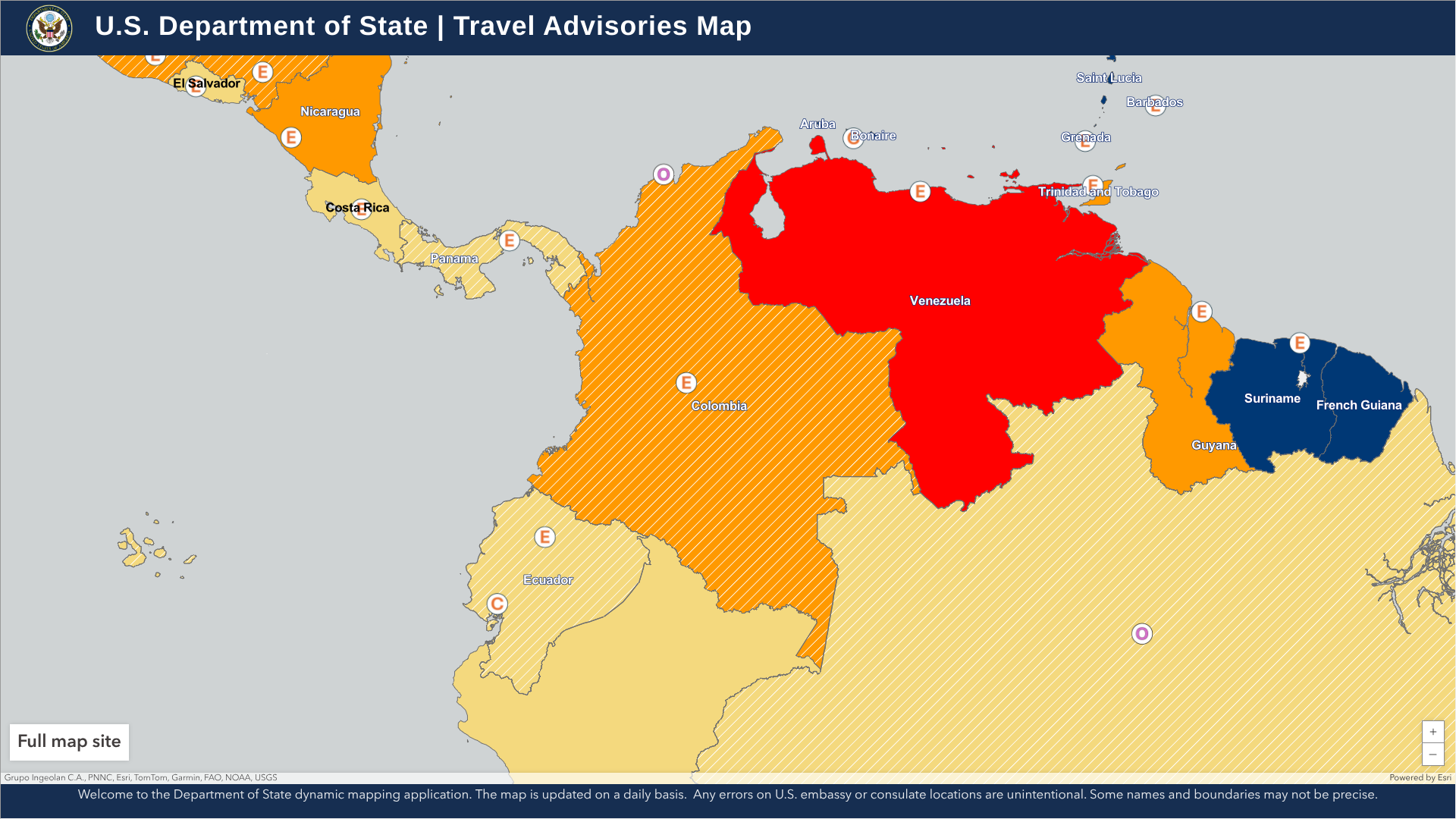
According to recent travel advisories issued by the U.S. government, it is advisable to delay trips to the Republic of Colombia in April 2025 due to ongoing crime and an unexpected yellow fever outbreak.
On April 17, 2025, the U.S. Department of State updated its Level 3: Reconsider Travel advisory for Colombia, citing ongoing civil unrest in various areas of the South American country.
The State Department has confirmed that visitors should not travel to the Arauca, Cauca (excluding Popayán), and Norte de Santander departments due to concerns about crime and terrorism.
Additionally, do not travel to this area for any reason to the Colombia-Venezuela border region, which is affected by crime, kidnapping, conflict between armed groups, and the risk of detention is elevated.
If you decide to travel to Colombia, the U.S. suggests enrolling in the Smart Traveler Enrollment Program to receive digital alerts from the local Embassy in Bogota and make it easier to locate you in an emergency.
From a health perspective, Colombia's current yellow fever (YF) outbreak has raised public health concerns as cases have been reported in areas where the disease had not historically occurred, including in Caldas.
To alert residents and visitors, Columbia's National Government declared a YF public health emergency on April 17, 2025.
In 2025, YF cases are distributed in nine departments: Tolima (59 cases), Putumayo, Nariño, Caquetá, Huila, Vaupés, Cauca, Meta, and Caldas. The total number of confirmed YF-related deaths in Tolima is 23.
According to the U.S. Centers for Disease Control and Prevention (CDC), yellow fever is a vaccine-preventable disease. Sanofi Pasteur YF-VAX® vaccine is commercially available at travel clinics and pharmacies in the United States.
And vaccination certificates, which are required for entry into designated countries, are issued upon completion.
The U.S. Department of Health and Human Services (HHS) recently issued a 'Stop Work Order' for GeoVax's investigational COVID-19 vaccine, GEO-CM04S1, which was being developed under a government-sponsored initiative.
According to GeoVax's press release on April 16, 2025, the U.S. Biomedical Advanced Research and Development Authority (BARDA) has decided to withdraw its funding, originally granted under Project NextGen (PNG), a $5 billion effort started under the previous U.S. administration to support the development of next-generation vaccines and therapies.
Of note, GEO-CM04S1 was the only multi-antigen/polyvalent COVID-19 vaccine candidate selected under the PNG initiative.
"While the recent HHS/BARDA Stop Work Order action was disappointing and surprising, our commitment to protecting vulnerable populations remains unchanged, and our clinical momentum is strong in support of our ongoing Phase 2 GEO-CM04S1 programs," commented David Dodd, Chairman and CEO of GeoVax.
GeoVax remains committed to addressing the critical medical need for GEO-CM04S1, particularly among the more than 40 million immunocompromised Americans and over 400 million people globally who remain inadequately protected by the current authorized COVID-19 vaccines.

Chikungunya, an arboviral disease previously unknown in the Americas, became an epidemic in the Region after its first indigenous case was reported in December 2013.
Since that time, the mosquito-transmitted virus has spread throughout most of the Region, and remains a key factor when planning summer vacations in 2025.
As of April 20, 2025, the Pan American Health Organization has confirmed 103,659 chikungunya cases and two related fatalities. Last year, over 420,000 cases and 236 fatalities were reported in the Region.
The U.S. Centers for Disease Control and Prevention (CDC) lists countries and territories with evidence of human-to-human transmission within the last five years.
The U.S. CDC states that most people infected with the chikungunya virus develop symptoms. People at risk for severe disease include newborns infected around the time of birth, older adults, and people with medical conditions.
To prevent this disease, effective chikungunya vaccines have been approved by the CDC and the U.S. FDA, and are commercially available at travel clinics and pharmacies as of 2025.

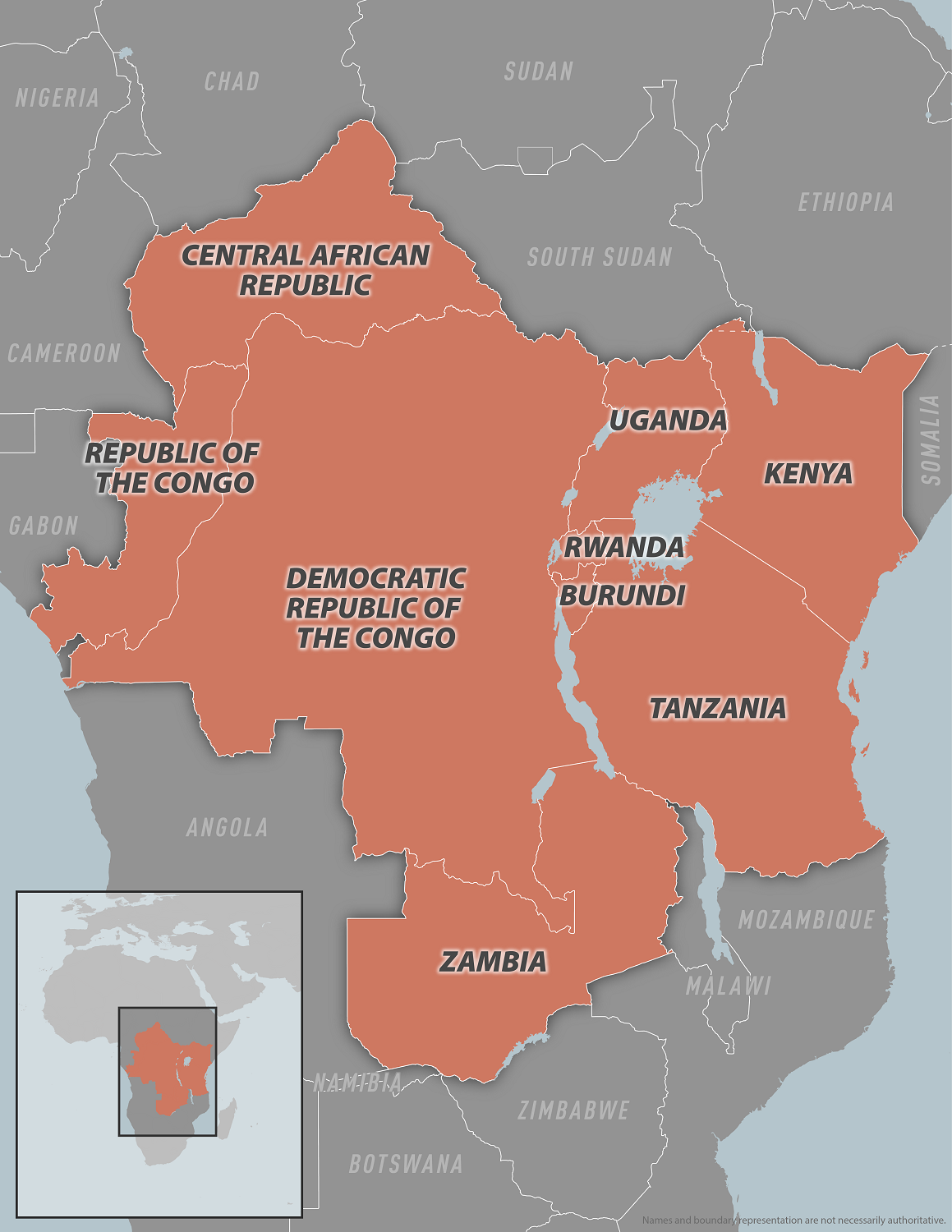
Since the declaration of the emergency, both regional and global support has increased, particularly for the Democratic Republic of the Congo (DRC), the epicentre of the ongoing mpox outbreak. Despite progress, significant challenges remain.
In the first two months of 2025, 60 countries reported mpox, with the majority of cases and deaths reported from the African continent.
The Africa CDC and WHO Joint Continental Mpox Plan has guided efforts to reduce this outbreak, focusing on ten key pillars: coordination, risk communication and community engagement, disease surveillance, laboratory capacity, clinical management, infection prevention and control, vaccination, research, logistics, and maintaining essential health services.
As of April 20, 2025, mpox vaccination efforts are underway, with more than 650,000 doses administered in six countries, 90% of which have been administered in the DRC.
Overall, over a million doses have been delivered to 10 countries, with efforts ongoing to secure additional vaccine supplies.
The leading mpox vaccine, JYNNEOS® (MVA-BN®, IMVAMUNE®), is produced by Bavarian Nordic A/S.
Along with the Continental Response Plan for Africa, the WHO has updated the global strategic plan to curb – and, where feasible, to stop – human-to-human transmission of mpox. The joint Continental Response Plan is aligned with the global strategy.
To alert international visitors of the ongoing mpox risk in Africa, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions, Travel Health Advisory on April 1, 2025.
The CDC says people usually get mpox through intimate or close contact, including sex, with an infected person.
Mpox vaccination is recommended for people who anticipate sexual activities during travel to countries with ongoing person-to-person transmission of clade I mpox. In the U.S., the JYNNEOS vaccine is commercially available at clinics and pharmacies as of 2025.
ImmunityBio, a vertically integrated biotechnology company developing next-generation therapies and vaccines that enhance the natural immune system to combat cancers and infectious diseases, recently reported a significant increase in revenue.
On April 15, 2025, ImmunityBio announced that it earned net product revenue of approximately $16.5 million during the three months ended March 31, 2025, representing a 129% increase over the net revenue earned during the fourth quarter of 2024.
With the issuance of the permanent J-code (J9028) in January 2025, ImmunityBio has experienced increased sales momentum for ANKTIVA®, the first U.S. FDA-approved immunotherapy for non-muscle-invasive bladder cancer that activates natural killer cells, T cells, and memory T cells to elicit a long-duration response.
The Company's range of immunotherapy and cell therapy platforms, both individually and in combination, act to drive and sustain an immune response, to create durable and safe protection against disease.
ANKTIVA's triangle offense against cancer includes natural killer cells, T cells, and memory T cells.
ANKTIVA (N-803) (nogapendekin alfa inbakicept-pmln) is a first-in-class interleukin-15 agonist IgG1 fusion complex consisting of an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha, which binds with high affinity to IL-15 receptors on NK, CD4+, and CD8+ T cells.
As of April 2025, the ANKTIVA plus BCG vaccine became available for treating bladder cancer patients at various clinical sites in the United States.