Search API
According to the World Health Organization (WHO), more than 13 million dengue cases were reported in 2024, the highest number on record.
According to recent data, the dengue global outbreak has continued in 2025.
To reduce the health impact of this mosquito-transmitted viral infection, more people than ever were immunized with a dengue vaccine.
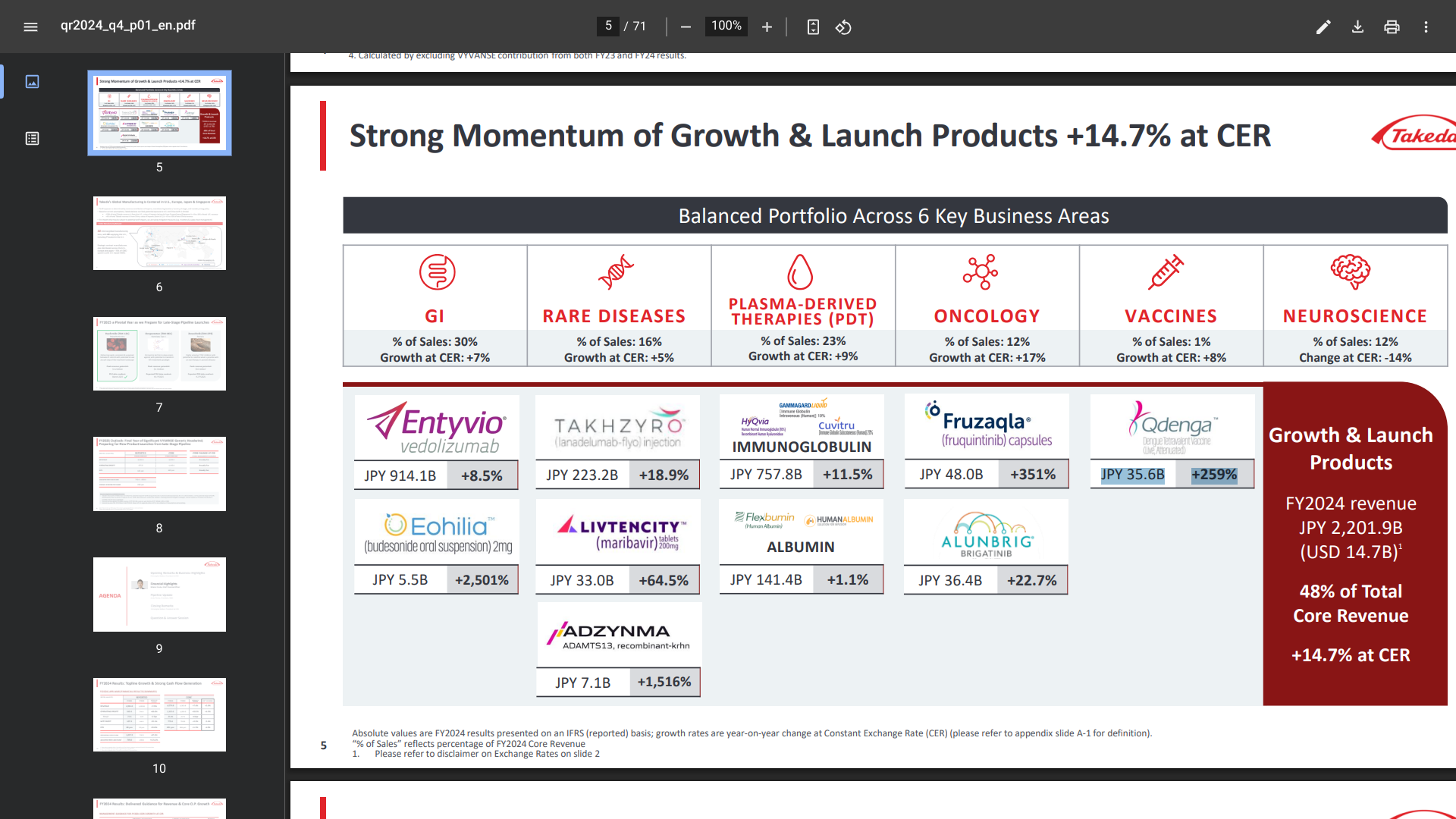
Announced today by Japan-based Takeda, the only WHO-listed dengue vaccine QDENGA®'s Q4 FY2024 (April 2024-March 2025), Revenue was JPY 35.6 billion ($247,746,985), an increase of 259%.
QDENGA® (TAK-003) is a tetravalent, two-dose vaccine approved for preventing dengue fever and/or Severe Dengue caused by any of the four serotypes of the dengue virus.
As of May 8, 2025, QDENGA was authorized in about 40 countries, with authorizations pending in the Philippines and India.
Unfortunately, no dengue vaccine is available in the United States, even in Puerto Rico, were dengue has become endemic.
A large-scale, population-based cohort study with a long-term follow-up recently investigated the association between live zoster (shingles) vaccination and the risk of various cardiovascular events.
A study published in the European Heart Journal on May 5, 2025, found a 23% lower risk of cardiovascular events in recipients of the shingles vaccine in the eight years following vaccination, with the most significant reduction observed 2–3 years post-vaccination.
The decrease in cardiovascular disease risk was more pronounced among males, individuals aged less than 60 years, those with unhealthy lifestyle habits, and those from low-income households and rural residents.
"A shingles infection can cause blood vessel damage, inflammation, and clot formation that can lead to heart disease," said study author Dong Keon Yon, PhD, from the Kyung Hee University College of Medicine, Seoul, in a press release.
"By preventing shingles, vaccination may lower these risks."
These findings suggest that live zoster vaccination may be beneficial for reducing the burden of cardiovascular disease in the general population.

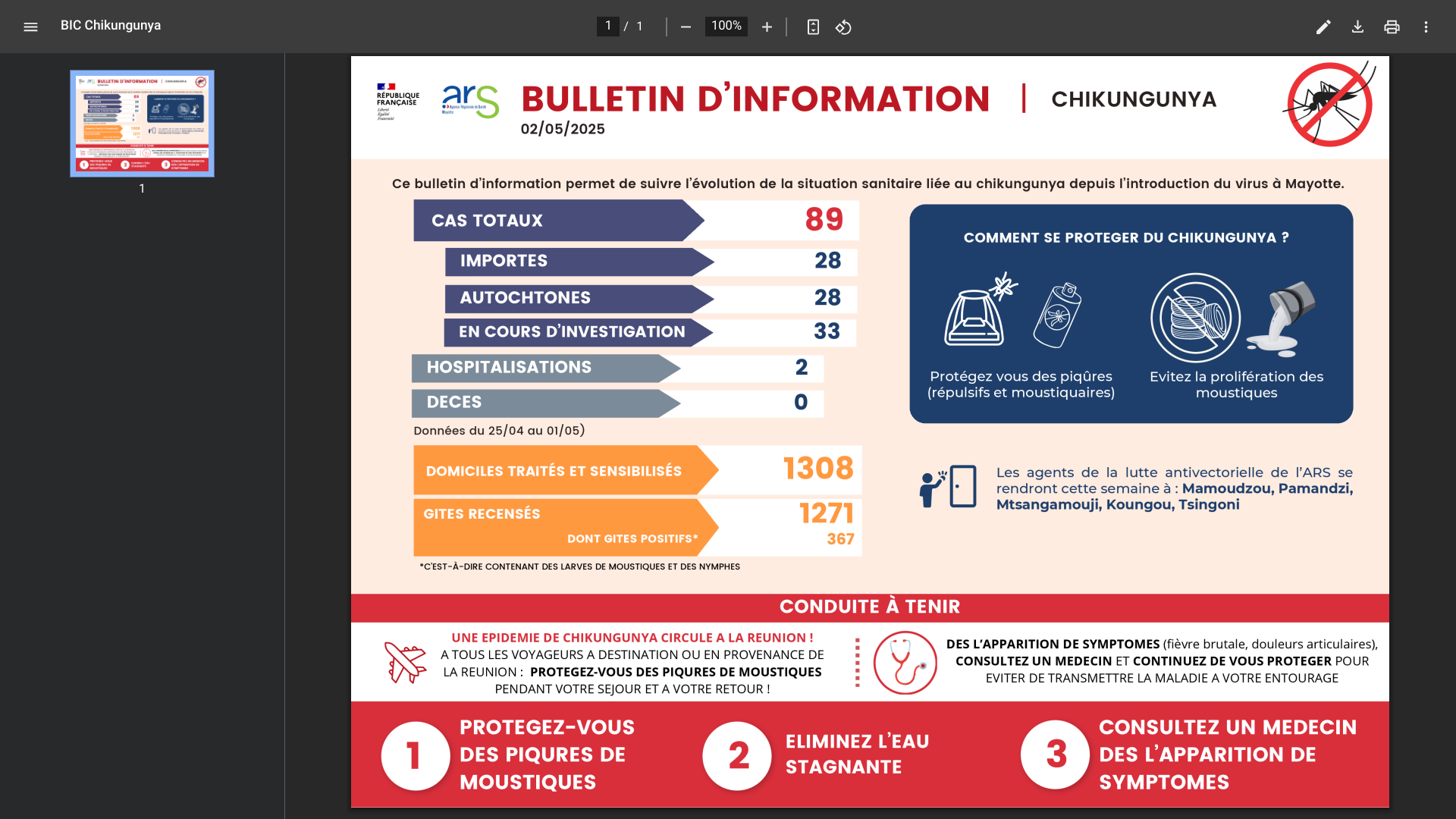
France's ARS Regional Health Agency continues to report chikungunya fever outbreaks in its Departments located east of Africa and Madagascar.
According to the information bulletin released by the Regional Health Agency on May 2, 2025, there have been 89 recorded cases in the Mayotte Department, a significant increase from 32 cases confirmed just two weeks ago.
Mayotte's outbreak breakdown includes 28 imported cases, 28 indigenous cases, and 33 cases currently under investigation. Additionally, two patients have been hospitalized.
The Regional Health Agency indicates that this health risk to the general population and any international visitors is high, and the outbreak could continue for weeks.
Since chikungunya is a vaccine-preventable disease, health authorities announced last week that adults aged 65 and younger will be included in the vaccination campaign in Mayotte.
A recent study found that a disease-blocking vaccine with 75% efficacy, if administered to 40% of individuals aged 12 years and older over three months, could have prevented approximately 34,200 chikungunya cases and 73 related fatalities.
In addition to chikungunya, Mayotte's cholera transmission are localized to Koungou, M'tsangamouji, and Mamoudzou. Mayotte increased its use of the cholera vaccine DUKORAL in the first quarter of 2025.
As of May 6, 2025, the U.S. CDC has not issued a Travel Health Advisory for visiting Mayotte.
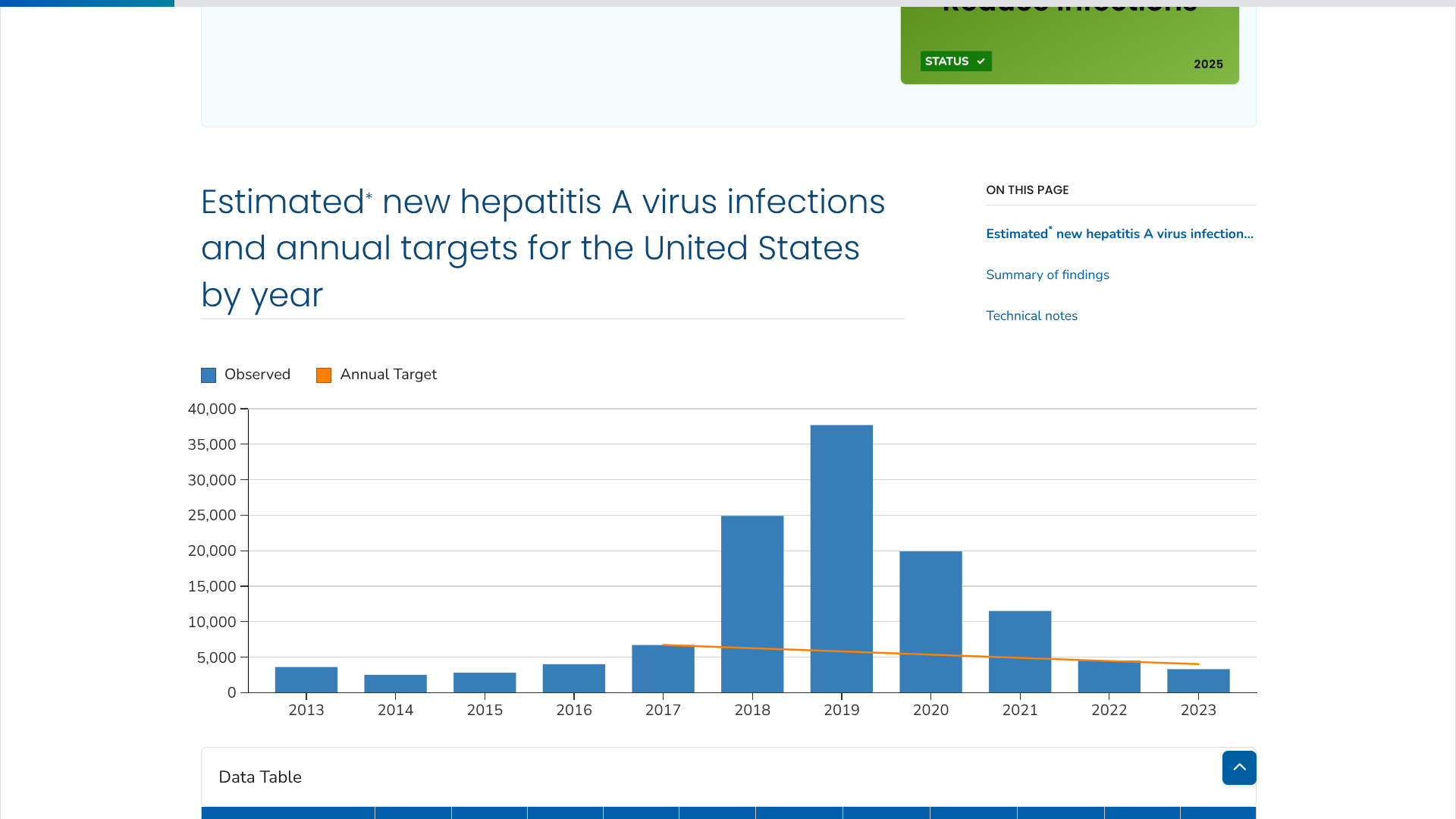
The Los Angeles County Department of Public Health (LAC DPH) has declared a communitywide outbreak of hepatitis A virus (HAV), a vaccine-preventable disease.
Since January 2024, 167 cases have been confirmed in LAC residents, including 29 in 2025. Typically, 30-50 cases are confirmed per year.
According to the U.S. CDC., the number of estimated new HAV infections increased annually in the U.S. beginning in 2015, peaked in 2019, and sharply decreased in 2020. This downward trend continued through 2023, reaching 3,300 estimated infections.
As of May 5, 2025, most LAC hepatitis A cases have occurred in people without typical risk factors such as travel, unstable housing, or illicit drug use.
Hepatitis A vaccine is widely available at primary care provider offices, pharmacies, and participating community sites serving uninsured people. It is also available at no cost at LAC DPH clinics. And private health insurance plans should cover the hepatitis A vaccine at no cost to beneficiaries.
While human-to-human transmission of avian influenza remains low, highly pathogenic strains such as H5N1 and H7N9 continue to pose serious global threats. According to the World Health Organization (WHO), if H5N1 mutates to enable human-to-human transmission, it could trigger a deadly pandemic.
Various avian influenza vaccines have been developed, and new, innovative technologies are being tested to address this global threat.
SK bioscience today announced that it has been selected for the Korea Disease Control and Prevention Agency (KDCA)- led initiative to develop vaccines against avian influenza, which has been identified as a high-risk candidate for the next pandemic.
Under the new program, SK bioscience and KDCA will co-invest approximately $3.7 million in early-stage development.
The company stated on May 6, 2025, that it will initiate development of a cell-culture-based avian influenza vaccine and aim to enter Phase 1/2 clinical trials in the second half of 2026.
The company says cell-culture-based vaccines offer greater effectiveness in pandemic response than traditional egg-based vaccines. Egg-based vaccines can face significant challenges during avian influenza outbreaks, as mass culling of poultry may limit access to uninfected fertilized eggs, making timely and large-scale vaccine production difficult and less responsive to emerging viral mutations.
In contrast, cell-culture-based vaccines are produced using animal cells in advanced aseptic facilities, minimizing the risk of contamination or infection. This method enables rapid, large-scale manufacturing and quicker adaptation to evolving virus strains.
SK bioscience has already utilized its cell-culture platform to develop vaccines against various viral diseases.
In the United States, various avian influenza vaccines have been supported by the U.S. FDA over the past few years.

The Brussels-based European Union (EU) and France today announced $566.6 million worth of incentives to lure scientists to the continent.
According to local media on May 5, 2025, the funds will support research projects and help universities cover the cost of recruiting foreign scientists.
"We call on researchers worldwide to unite and join us," French President Emmanuel Macron said at Paris' Sorbonne University alongside European Commission President Ursula von der Leyen.
The EU's President Von der Leyen also wants its member states to invest 3% of their gross domestic product in research and development by 2030.