Search API
Numerous studies have indicated an increased risk of stroke and myocardial infarction (MI) following herpes zoster (HZ); however, the impact of vaccination remains uncertain, wrote researchers in a global analysis.
To assess the effectiveness of HZ (shingles) vaccination with recombinant zoster vaccine (RZV) or zoster vaccine live-attenuated (ZVL) against cardiovascular (CV) events in adults, numerous phase 3 and observational studies were assessed.
Across these studies, any HZ vaccination (RZV or ZVL) was associated with a significantly lower risk of stroke and MI, versus no HZ vaccination.
The pooled RR of 0.82 (95% CI 0.76–0.87) in adults ≥18 years and RR of 0.84 (0.82–0.87) in adults ≥50 years.
Thus, vaccine effectiveness was 18% (13–24%) and 16% (13–18%) in preventing CV events, respectively.
RZV vaccination was associated with a significantly lower risk of stroke and MI versus no HZ vaccination: pooled RR 0.79 (0.65–0.97) in adults ≥18 years and RR 0.79 (0.64–0.97) in adults ≥50 years, with a vaccine effectiveness of 21% (3–35%) and 21% (3–36%), respectively.
These researchers concluded that HZ vaccination was associated with a significantly lower rate of cardiovascular events.
Study author Dr. Charles Williams, Global Associate Medical Director for Vaccines at GSK, commented in a media release, "Further research studies are now needed to find out whether this association can be attributed to an effect of herpes zoster vaccination."
This assessment is scheduled to be presented on August 30, 2025.
As of August 29, 2025, shingles vaccination services are offered at most pharmacies and clinics in the United States and the United Kingdom.

With about one million people developing herpes zoster (shingles) in the US each year, simplifying the vaccination process is essential for at-risk seniors.
Approximately 99% of older adults have the virus that causes shingles inside their bodies.
With 55 million Americans aged 65 or older, many of whom live in California, Florida, and Texas, accessing the US Food and Drug Administration (FDA)-approved Shingrix® vaccine is a priority.
GSK plc, the manufacturer of Shingrix, announced that the FDA has recently approved a prefilled syringe presentation, which eliminates the need to reconstitute the vaccine separately before administration, thereby simplifying the vaccine administration process for healthcare professionals.
Brigid Groves, Vice President of Professional Affairs at the American Pharmacists Association, stated in a press release on July 17, 2025, "The prefilled syringe presentation of GSK's shingles vaccine is good news, providing a convenient method of administration."
"The FDA approval is a positive step toward driving prevention of this painful disease, and as a practicing pharmacist, I welcome the availability of this new presentation."
The Centers for Disease Control and Prevention (CDC) recommends two doses of GSK's shingles vaccine to prevent shingles and related complications in adults aged 50 years or older. Additionally, two doses are recommended for adults aged 19 years or older who are or will be immunocompromised or immunosuppressed.
The CDC states that as people age, the strength of their immune system's response to infection wanes, increasing the risk of developing shingles.
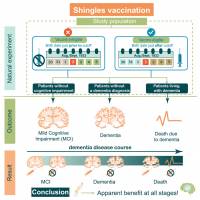
Additionally, Shingrix vaccination may play a role in delaying the onset of dementia.
Over the past few years, various studies have reported causal evidence that herpes zoster vaccination may prevent a proportion of dementia cases.

Over the past decade, numerious herpes zoster cases were prevented with two doses of a U.S. FDA-approved vaccine that contains an adjuvant. According to new research, there may be an additional, measurable benefit from vaccination.
A Brief Communication published by NPJ Vaccines on June 25, 2025, reported a lower risk of dementia associated with AS01-adjuvanted vaccination against shingles.
In propensity-score matched cohort studies involving 436,788 individuals, both the AS01-adjuvanted shingles and respiratory syncytial virus vaccines, administered individually or in combination, were associated with a reduced risk of dementia at 18 months.
AS01 may protect against dementia through specific immunological pathways.
In particular, stimulation of toll-like receptor 4 with monophosphoryl lipid A (MPL; one of the components of the AS01 system) has been shown to improve Alzheimer’s disease pathology in mice.
In addition, the two main ingredients of AS01, MPL and QS-21 (a purified plant extract derived from Quillaja saponaria), act synergistically to activate macrophages and dendritic cells, triggering an age-independent cytokine cascade that culminates in the production of interferon gamma (IFN-γ).
IFN-γ might attenuate amyloid plaque deposition (as seen in mice) and is negatively correlated with cognitive decline in cognitively unimpaired older adults.
These neuroprotective mechanisms may reach their full potential at or below the dose of AS01 administered within a single vaccine, so that administering both the AS01 shingles and RSV vaccines does not provide any additional benefits.
This saturation effect could also explain why the level of protection against dementia appears similar between the AS01 shingles vaccine (which is given in two doses) and the AS01 RSV vaccine (administered as a single dose).
No difference was observed between the two AS01-adjuvanted vaccines, suggesting that the AS01 adjuvant itself plays a direct role in reducing the risk of dementia.
A previous study found similar cross-protection benefits.
In July 2024, a University of Oxford-led study concluded that receiving the recombinant Shingrix® vaccine was associated with a 17% increase in diagnosis-free time, translating into 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As of July 7, 2025, shingles vaccination services are offered at most pharmacies in the United States.

Recent research suggests that a herpes zoster (HZ) vaccine, commonly referred to as the shingles vaccine, may reduce the risk of receiving a dementia diagnosis following vaccination.
According to a study published in JAMA on April 23, 2025, there is evidence of a beneficial effect of herpes zoster vaccination in preventing or delaying dementia, which is more likely to be causal than the associations reported in existing correlational evidence.
In this quasi-experimental study using electronic health record data from Australia, being eligible for herpes zoster vaccination based solely on date of birth significantly decreased the probability of receiving a new dementia diagnosis during 7.4 years by 1.8 percentage points.
A similar study conducted in Wales also showed that HZ vaccination appears to prevent or delay the onset of dementia by about 20%.
These researchers wrote, 'this study and the analysis in Wales provide evidence that is more robust to confounding concerns (eg, healthy vaccinee bias) than is the existing associational evidence.'
In the United States, shingles vaccination services are offered at most pharmacies in April 2025.

- 1 of 11
- next ›