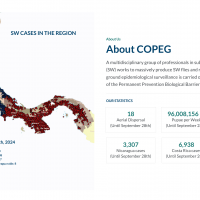
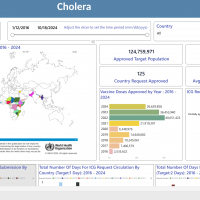
Search API
With the global expansion of the chikungunya, dengue, and zika viruses in 2024, many publishers are challenged to present outbreak data in a balanced voice and tone in the media.
The lessons learned from the recent pandemic indicate that people want to read the data as soon as possible, whether published as a pre-print or peer-reviewed. With new information, people can privately confer with their healthcare providers about personal health actions such as vaccination.
A recent Editorial published by The Lancet Infectious Diseases on October 15, 2024, encapsulated these concerns regarding the sudden Oropouche virus outbreak in the Region of the Americas, such as in Brazil and Cuba.
These researchers wrote, "While such early data are key to understanding and tackling outbreaks, they come with considerable uncertainty and raise questions about how to report and publish them responsibly."
"However, we believe knowledge will inform public health responses, focus resources, and garner urgency in studying the virus further, which is the first step to prevention and treatment."
The full, unedited Editorial is posted at this link.

Blue Lake Biotechnology, Inc. and its affiliate CyanVac LLC today announced the presentation of positive interim pediatric clinical data on BLB201, Blue Lake’s vaccine candidate against respiratory syncytial virus (RSV).
Hong Jin, Ph.D., Chief Scientific Officer of Blue Lake and CyanVac, presented a talk that included results from an interim analysis of RSV-seropositive children participating in the company’s ongoing Phase 1/2a pediatric study.
The data demonstrated the safety and immunogenicity of BLB201 in this population, including RSV F-specific systemic and mucosal antibody responses and cell-mediated immune responses in CD4+ and CD8+ T cells.
“Our vaccine approach is designed to stimulate potent immune responses from the humoral, cellular, and mucosal pillars of the immune system,” said Biao He, Ph.D., CEO of Blue Lake and CyanVac, in a press release on October 21, 2024.
“Many currently available vaccine technologies such as mRNA and protein-based vaccines are ineffective in inducing mucosal immunity because they are given intramuscularly."
"By robustly stimulating all three pillars of immunity, our intranasal vaccine may prevent disease transmission and generate longer-lasting immunity than other types of vaccines.”
The U.S. Government has begun health screening air travelers who have been to the Republic of Rwanda in the last 21 days. This action is related to the recent Marburg virus outbreak in which 15 people have died.
Virgin Atlantic Airways confirmed that effective October 16, 2024, these Marburg virus-related screenings are a precautionary measure and will take place in Washington Dulles, Chicago O'Hare, and New York JFK.
This means any customer who has recently visited Rwanda will be rerouted through one of these airports to facilitate these mandatory screenings.
The Company says if you have booked directly with Virgin Atlantic and have been to Rwanda, don't hesitate to contact us to discuss your options.
As of October 19, 2024, Marburg vaccine candidates are being evaluated in various clinical trials, but the U.S. FDA has approved no vaccine.

The Federal Republic of Nigeria’s Federal Ministry of Health has been leading critical malaria control interventions, including introducing the R21/Matrix-M™ malaria vaccine as part of the country’s comprehensive strategy to combat the disease.
“The arrival of (800,000) malaria vaccines is a monumental step in our national efforts to reduce malaria morbidity and mortality,” said Professor Muhammad Ali Pate, Coordinating Minister of Health and Social Welfare, in a press release on October 17, 2024.
This announcement is critical to Nigerians.
According to the 2023 World Malaria Report, nearly 200,000 deaths from malaria occurred in Nigeria. In some regions, such as Kebbi State, this malaria prevalence rate is as high as 49%.
The malaria vaccine, which requires four doses, will be administered to children under one year of age as part of Nigeria’s Routine Immunization schedule. The first phase of the rollout will begin in Kebbi and Bayelsa States in November 2024.
On October 2, 2023, the World Health Organization recommended R21/Matrix-M to prevent malaria in children. Since then, it has been offered in various African countries.
The U.S. CDC says malaria vaccines reduce uncomplicated malaria by ~40%, severe malaria by ~30%, and all-cause mortality by 13%.
Neither approved malaria vaccine is available in the United States, but the CDC recommends that travelers going to Nigeria take prescription medicine to prevent malaria.
Additionally, the CDC has issued travel advisories for Nigeria's yellow fever, measles, polio, and diphtheria outbreaks.

When Johnson & Johnson (J&J) announced third-quarter earnings this week, its growth exceeded Wall Street’s expectations.
However, based on J&J's announcement on October 4, 2024, its future results will not include a Dengue product.
J&J confirmed the discontinuance of the Phase 2 field study to evaluate the efficacy of the investigational antiviral candidate mosnodenvir in preventing Dengue virus in adults. The decision to discontinue this study is part of a strategic reprioritization of the Company’s Communicable Diseases research and development portfolio.
This unfortunate news will bring essential focus to second-generation dengue vaccines and innovative vaccine candidates conducting late-stage clinical research in 2024, such as Butantan Institute's Butantan-DV tetravalent dengue vaccine.
As of October 18, 2024, Dengue's global outbreak continues unabated.