Shigellosis Outbreaks
Shigellosis Outbreaks May 2025
The World Health Organization (WHO) says Shigellosis, a gastrointestinal infection caused by one of four species of Shigella bacteria, continues to pose a significant public health problem and remains endemic in many developing countries in 2025. Among Shigella species, Shigella dysenteriae type 1 (Sd1) represents a particular threat because of the severity of the disease it causes.
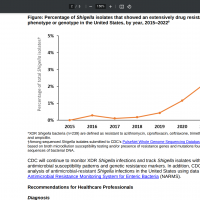
The U.S. Centers for Disease Control and Prevention (CDC) confirmed in May 2025 that the Shigella bacteria can spread quickly. The most common ways people get sick are by eating or drinking contaminated food or water, and by contact with someone with a Shigella infection. People with Shigella infection can shed the bacteria in their stool for weeks after symptoms have dissipated. The CDC Yellow Book 2024 says extensively drug-resistant (XDR) Shigella infections were 5% of Shigella infections (Shigellosis) reported in 2022, compared with 0% in 2015. These articles provide additional information about previous Shigellosis outbreaks.
Shigellosis Outbreaks in the United States
The CDC estimates that Shigellosis cases are the third most common bacterial enteric disease in the U.S. As of March 8, 2025, the CDC reported 2,352 Shigellosis cases in the U.S. in 2025. As of week #52, ending December 28, 2024, the CDC confirmed 5,126 Shigella cases, led by California (4,365) and New York (2,990). The Northern Nevada Public Health identified a Shigellosis outbreak of at least 14 cases and nine hospitalizations at the end of 2024. In 2023, the CDC reported 17,176 cases in the U.S.
A Brief Report published by AJIC on December 9, 2024, describes a mutation of Shigella sonnei, a strain of Shigella bacteria resistant to five of the antibiotic classes most commonly prescribed for such infections. In Los Angeles, CA, Dr. Shangxin Yang described a mutation of Shigella sonnei, a strain of Shigella bacteria resistant to five of the antibiotic classes most commonly prescribed for such infections. These cases had a distinct genetic mutation that made the bacteria resistant to another class of antibiotics, the cephalosporins. The strain appears to be unique to LA County. XDR Shigella cases have increased since they were first detected in California in 2017. By 2022, 3.2% of Shigella isolates were XDR, which increased to 6.8% in 2023. From January through May 2024, 12% (118/978) of California Shigella isolates were reported as XDR. Shigella sonnei isolates accounted for the most significant percentage (78%), followed by Shigella flexneri (22%).
Shigellosis Outbreaks in Africa
A review published in October 2024 highlights the burden of Shigellosis in Africa. S. flexneri remains the most prevalent species associated with shigellosis cases, with S. sonnei being the second most dominant. The overall pooled estimate of Shigella prevalence was 5.9% (95% CI: 4.9 – 7.0%). The antimicrobial resistance patterns observed in the study suggest local antimicrobial patterns when choosing antibiotics to treat Shigellosis.
Shigellosis Outbreaks in the Region of the Americas
The Pan American Health Organization (PAHO) issued an Epidemiological Alert in 2022 regarding the spread of Shigella sonnei with extreme antibiotic resistance and the potential risk to Latin America and the Caribbean.
Shigellosis Outbreaks in Europe
In 2024, the ECDC reported that 30 EU/EEA countries reported 4,149 confirmed cases of Shigellosis in 2022. Three countries accounted for 50.6% of all cases: France, the Netherlands and Spain. Of those cases with information on travel history, 48% were associated with travelers. For 2021, 30 EU/EEA countries reported 2,115 confirmed cases of Shigellosis.
Extensively Drug-Resistant Shigella Outbreaks
In Vancouver, Canada, Shigella sonnei has caused sexually transmitted enteric infections in men who have sex with men. A study recently observed a high rate of multidrug-resistant (MDR) S. sonnei bacteremia among persons experiencing homelessness.
Shigellosis Vaccine
As of May 2025, the U.S. FDA has not approved any shigellosis vaccine candidates. However, in November 2024, a Phase 2b controlled human infection model study of Shigella4V2, the world’s most clinically advanced tetravalent bioconjugate shigellosis vaccine candidate, was launched.