Search API
Stanford University scientists recently reported findings in a mouse study that could lead to a needle-free vaccination approach that eliminates reactions such as fever, swelling, and pain.
Published in the journal Nature on December 11, 2024, Fischbach and colleagues stated the ubiquitous skin colonist Staphylococcus epidermidis elicits a CD8+ T cell response pre-emptively in the absence of an infection.
They wrote that this colonist also induces a potent, durable, and specific antibody response that is conserved in humans and non-human primates.
These specialized proteins can stick to specific biochemical features of invading microbes, often preventing them from getting inside cells or traveling unmolested through the bloodstream to places they should not go.
The initial experiments included dipping a cotton swab into a vial containing S. epidermidis. Rub the swab gently on the head of a regular mouse — no need to shave, rinse, or wash its fur — and put the mouse back in its cage. Draw blood at defined time points over six weeks, asking: Has this mouse’s immune system produced antibodies that bind to S. epidermidis?
The mice’s antibody response to S. epidermidis was “a shocker,” Fischbach said.
“Those antibodies’ levels increased slowly, then some more — and then even more.” At six weeks, they’d reached a higher concentration than expected from a regular vaccination — and they stayed at those levels.
“It’s as if the mice had been vaccinated,” Fischbach said. Their antibody response was as strong and specific as if it had been reacting to a pathogen.
“The same thing appears to be occurring naturally in humans,” Fischbach said. “We got blood from human donors and found that their circulating levels of antibodies directed at S. epidermidis were as high as anything we get routinely vaccinated against.”
That’s puzzling, he said: “Our ferocious immune response to these commensal bacteria loitering on the far side of that all-important anti-microbial barrier we call our skin seems to have no purpose.”
“We think this will work for viruses, bacteria, fungi, and one-celled parasites,” he said. If things go well, he expects this vaccination approach to enter clinical trials within two or three years.
The completed, unedited Stanford news article written by Bruce Goldman is posted at this link.
Sinovac Biotech Ltd. today announced the enrollment for a Phase Ⅲ clinical trial on a bivalent vaccine candidate to prevent Hand, Foot, and Mouth Disease (HFMD) caused by both Enterovirus 71 (EV71) and Coxsackievirus 16 (CA16).
As of December 16, 2024, no multivalent vaccines against HFMD have been approved for marketing worldwide.
SINOVAC is also developing a vaccine to prevent HFMD caused by EV71, CA16, CA10, and CA6.
According to the U.S. CDC, non-polio enteroviruses cause about 10 to 15 million infections and tens of thousands of hospitalizations each year in the United States.
HFMD can be caused by several enteroviruses, which often exhibit low cross-immunogenicity, leading to insufficient protection. HFMD is very contagious and mainly affects children under 5 years old, accounting for at least 90% of the total HFMD patients. Most people recover on their own in 7 to 10 days.

To ensure the United States is well stocked with Anthrax vaccines, the Biomedical Advanced Research and Development Authority (BARDA) today announced a $50 million option to Emergent BioSolutions Inc. for the acquisition of CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), a U.S. FDA approved vaccine.
Vaccine deliveries will begin in late 2024 and be complete by April 2025.
While Anthrax is rare in the U.S., occasional outbreaks happen in wild and domestic grazing animals such as cattle or deer, says the U.S. CDC.
Most people will never be exposed to any of the four types of Anthrax. However, jobs, hobbies, and activities can put some people at higher risk of exposure.
Furthermore, the U.S. government remains concerned about the exposure to Bacillus anthracis, the cause of Anthrax, which is a likely agent for bioterrorism. In 2001, letters with powdered anthrax spores were mailed in the U.S., causing 22 infections and five related fatalities.
“An anthrax emergency continues to be a significant public health threat due to its ability to be easily disseminated, lethality, and potential for widespread impact,” said Paul Williams, senior vice president, products head at Emergent, in a press release on December 16, 2024.
“This procurement award further bolsters anthrax preparedness and demonstrates Emergent’s commitment to public health preparedness.”
This new BARDA award follows a previously announced contract modification of $30 million to supply CYFENDUS.
Bavarian Nordic A/S today announced an agreement with Serum Institute of India Pvt. Ltd. ("SII") for its MVA-BN® mpox vaccine.
Under the agreement revealed on December 16, 2024, the companies will undertake a technology transfer of the current manufacturing process for MVA-BN (JYNNEOS®) to SII to enable the supply of the mpox vaccine for the Indian market. Furthermore, upon the relevant regulatory approvals, SII will perform contract manufacturing of MVA-BN, ensuring global access even during outbreaks of mpox.
SII's multifunctional production and one of the most extensive facilities in Pune, India, has an annual capacity of 4 billion doses.
In a press release, SII's CEO Adar Poonawalla said, "The recent mpox outbreak underscores the critical need for a swift and coordinated response. Partnering with Bavarian Nordic on the MVA-BN mpox vaccine reflects our shared commitment to protect millions at risk. Leveraging our manufacturing strength and rapid response capabilities, we aim to enhance epidemic preparedness and expand access to life-saving vaccines, safeguarding vulnerable populations and easing the global burden of mpox."
Bavarian Nordic continues to explore additional opportunities to establish partnerships, including with local African manufacturers, to ensure equitable access to MVA-BN.
Local media recently reported that since 2022, 33 confirmed cases of Mpox have been reported in India. And a Surveillance Program has been recently instructed to intensify surveillance of Mpox patients at airports. India confirmed its first case of the clade Ib mpox strain in late September 2024.
Since it was approved to protect people from smallpox in 2019, the JYNNEOS brand is now commercially available at many clinics and pharmacies.
From an efficacy perspective, U.S. CDC research published in the journal Emerging Infectious Diseases, Volume 30, Number 10—October 2024, concluded the estimated effectiveness of the 2-dose JYNNEOS vaccine was about 80% against clade 2.
A study announced in March 2024 showed that JYNNEOS antibodies waned within a year.
Vaccine efficacy data against clade I mpox is pending.

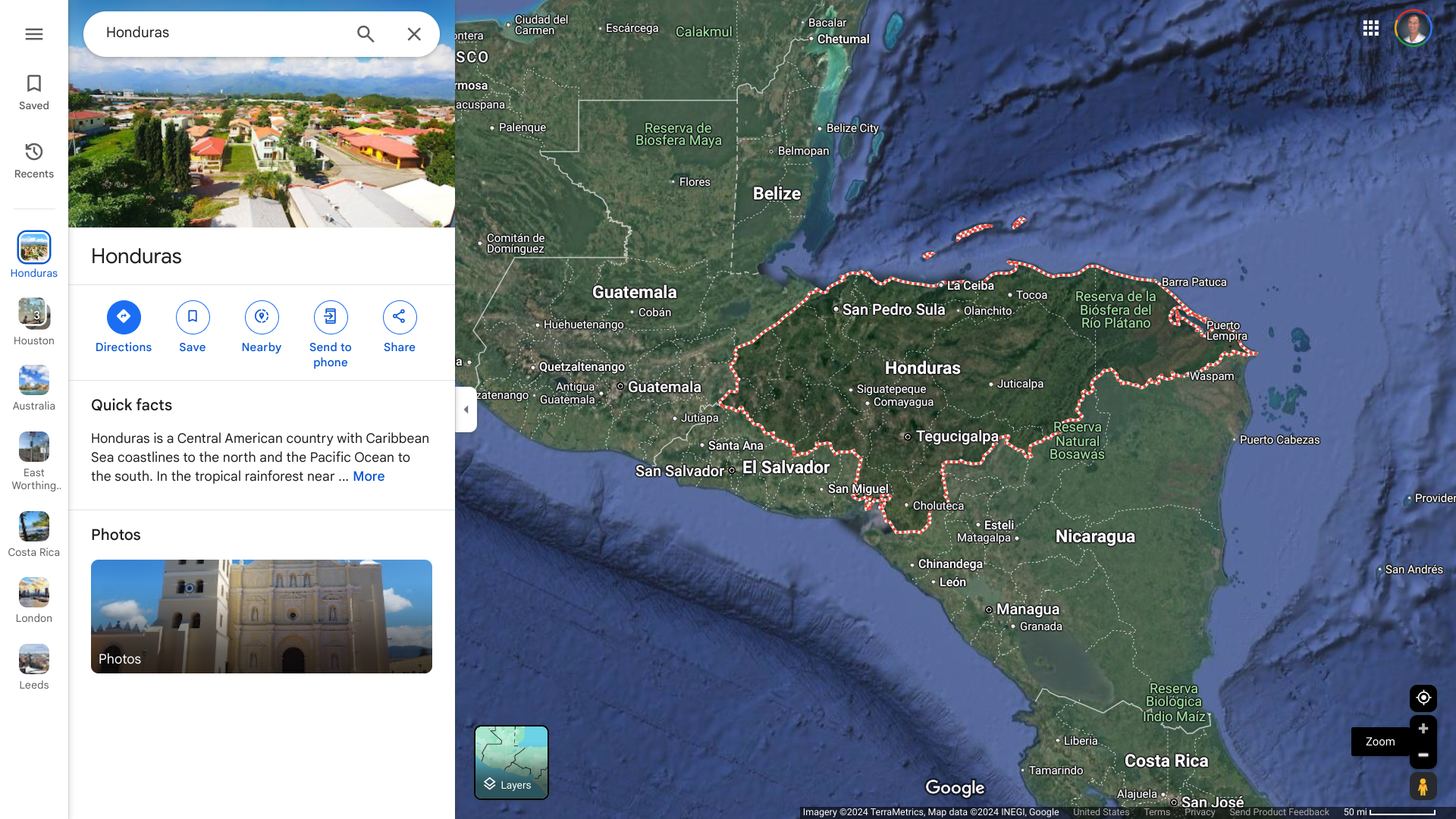
As many winter vacationers are finalizing plans to visit warm Central American beaches, the U.S. Department of State issued a high-level advisory for the Republic of Honduras.
As of December 10, 2024, the State Department's Level 3 advisory warns against traveling to the Gracias a Dios Department, Honduras's most eastern department, due to civil unrest. This department's infrastructure is weak, government services are limited, and police and military presence is scarce.
In December 2022, the Government of Honduras declared a "State of Exception." That declaration remains in effect in 2024 and has been modified to include more cities.
However, some locations in Honduras were found welcoming for visitors this winter.
The State Department's advisory states: "There is a concentration of resources around resort areas in the Bay Islands, which include Roatan, Utila, and Guanaja, and these areas are better policed."
If you visit Honduras, enroll in the Smart Traveler Enrollment Program to receive digital alerts and make locating you in an emergency easier. If U.S. citizens need assistance while in Honduras, the U.S. Embassy in Tegucigalpa is located at Avenida La Paz, Tegucigalpa, M.D.C.
From a health perspective, the Honduran Ministry of Health declared a national emergency in Honduras due to the increased risk of the mosquito-transmitted dengue virus in June 2024.
As of December 15, 2024, over 175,000 dengue cases and 153 related fatalities have been reported this year.
Additionally, Honduras has reported chikungunya and zika patients in 2024.
For disease prevention, an approved chikungunya vaccine (Valneva SE's IXCHIQ®) is available at many travel clinics and pharmacies in the U.S., but no vaccine is offered for dengue or zika.