Search API
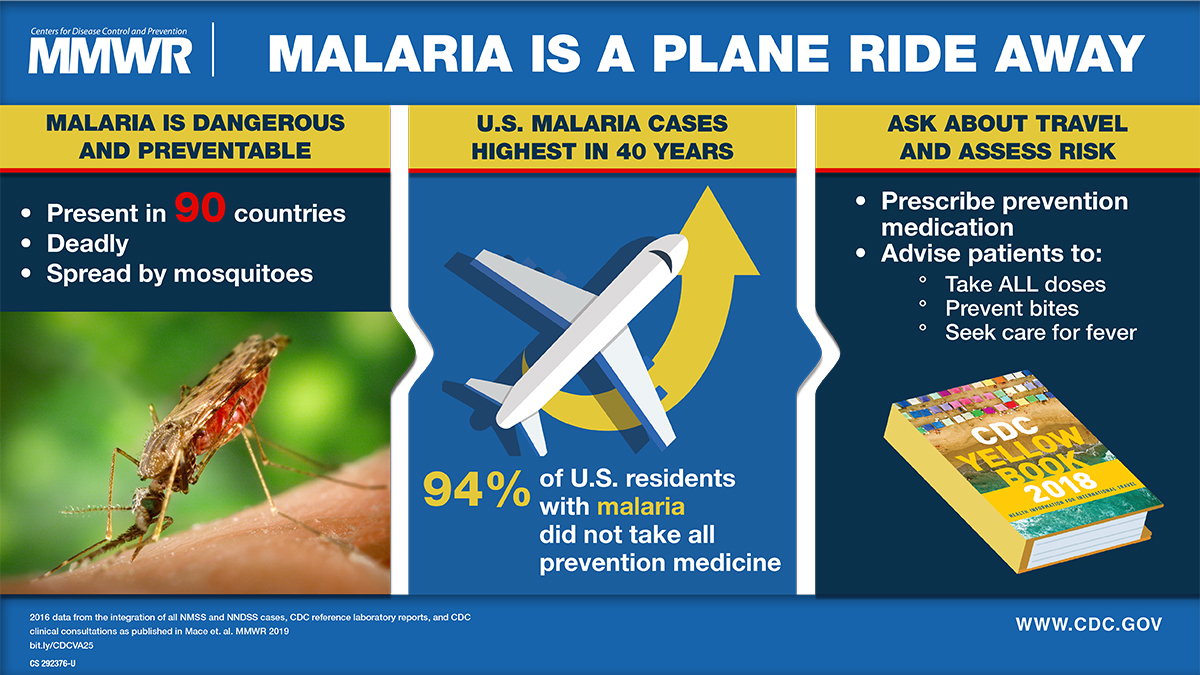
After a measurable decline in malaria cases globally over the last two decades, case numbers have rebounded throughout Africa during the previous two years, highlighting the need for enhanced prevention and treatment options.
According to the World Health Organization (WHO), despite the expenditure of $4 billion per year, malaria fatalities have not substantively been reduced during outbreaks.
While the WHO recommends two malaria vaccines (Mosquirix™ and R21 / Matrix-M™) to reduce mosquito-transmitted malaria outbreaks in Africa, a new study has identified a potential change in case management.
In The Lancet Infectious Diseases, Virak Eng and colleagues provide evidence of the benefit of high total-dose primaquine (7 mg/kg) compared with low total-dose primaquine (3·5 mg/kg) to prevent relapsing P vivax malaria in Cambodia.
These findings, published on March 17, 2025, and funded by the U.S. National Institutes of Health, provide strong evidence for the optimal primaquine dose for anti-relapse therapy and support the 2024 WHO malaria treatment guidelines update recommending high-dose primaquine in most endemic countries.
In the United States, most malaria cases are international travel-related, not locally transmitted.
Previously, the WHO estimated the annual global demand for malaria vaccines at 40–60 million doses by 2026. These vaccines are not commercially available in the U.S.
While a U.S. FDA-approved shingles vaccine has been well received in the market and numerous studies indicate it's effective and safe, a non-mRNA adjuvanted subunit vaccine candidate has completed a significant series B financing.
Announced on March 17, 2025, Curevo Vaccine closed a $110 million Series B round to advance the development of Amezosvatein, its vaccine for shingles (varicella-zoster virus).
"This Series B round will fund the extension of our successful Phase 2 program into an additional 640 participants, including the key population of adults over age 70, to finalize dose selection ahead of the Phase 3 program," said Curevo's CEO, George Simeon, MBA/MPH, in a press release.
"Designed based upon feedback from regulators and other stakeholders, this short extension trial will begin mid-2025 and set the company for clinical, strategic, and regulatory success."
The Phase 2 study (NCT05304351) 's primary completion date is March 31, 2025.
Like Shingrix®, amezosvatein, the assigned non-proprietary name for CRV-101, uses a subunit protein antigen called glycoprotein 'E' (gE). Targeting the gE antigen is proven to elicit a long‑term, protective immune response to prevent shingles.
Amezosvatein's adjuvant contains an optimized version of the TLR4 agonist proven by Shingrix to be biologically active in shingles vaccination.
Amezosvatein was engineered to maintain exceptional efficacy and have a best‑in‑class tolerability profile.
The SLA-SE adjuvant formulation was developed at Seattle‑based Access to Advanced Health Institute and amezosvatein was licensed from the Mogam Institute for Biomedical Research, a research institute funded by South Korea's GC Biopharma.
Until a phase 3 study is completed and approved by the FDA, this shingles vaccine candidate will not become commercially available in the U.S. Currently, the U.S. CDC recommends two doses of the recombinant zoster vaccine to prevent shingles and related complications in most people. This vaccine is offered at most pharmacies.
The Shingles Vaccine industry is projected to reach about $7 billion by 2032.

While most of the local media attention has been focused on the measles outbreaks in west Texas, the city of Houston actually reported Texas's initial two measles cases in January 2025.
Since then, the Houston Health Department (HDD) reported a third measles case involving an unvaccinated infant on March 16, 2025. This child was exposed to measles during international travel and is recovering at home.
HDD stated that this new case is unrelated to the earlier measles cases reported in Houston.
Dr. David Persse, Chief Medical Officer for the City of Houston, commented, "The best way to protect yourself and your loved ones from measles is through vaccination."
"Although measles was declared eliminated in the U.S. in 2000, international travel continues to present a risk," added Dr. Persse.
Houston and Harris County, TX, are home to about 5 million people, with two international airports serving millions of travelers annually. For more information on measles and vaccination options in Houston, including Harris County, visit HoustonHealth.org.
"The recent measles case in Houston highlights the continued importance of vaccination in protecting individuals and communities," V. Yvette Cheeks MSN, RN, NPE-C information Vax-Before-Travel News.
"As global travel presents ongoing risks, the Houston Health Department urges residents to confirm their measles immunity. Vaccines remain the most effective tool in preventing the resurgence of this highly contagious disease," added Cheeks, the CEO of Houston-based The Immunization Clinic.
Globally, the U.S. CDC has issued Travel Health Advisories that continue to identify measles outbreaks in 57 countries, including Canada, England, and Romania.
HDD and the CDC recommend that most people confirm their measles immunity before visiting outbreak areas like Texas or eastern New Mexico. The MMR vaccine is offered at travel clinics and pharmacies throughout the U.S.

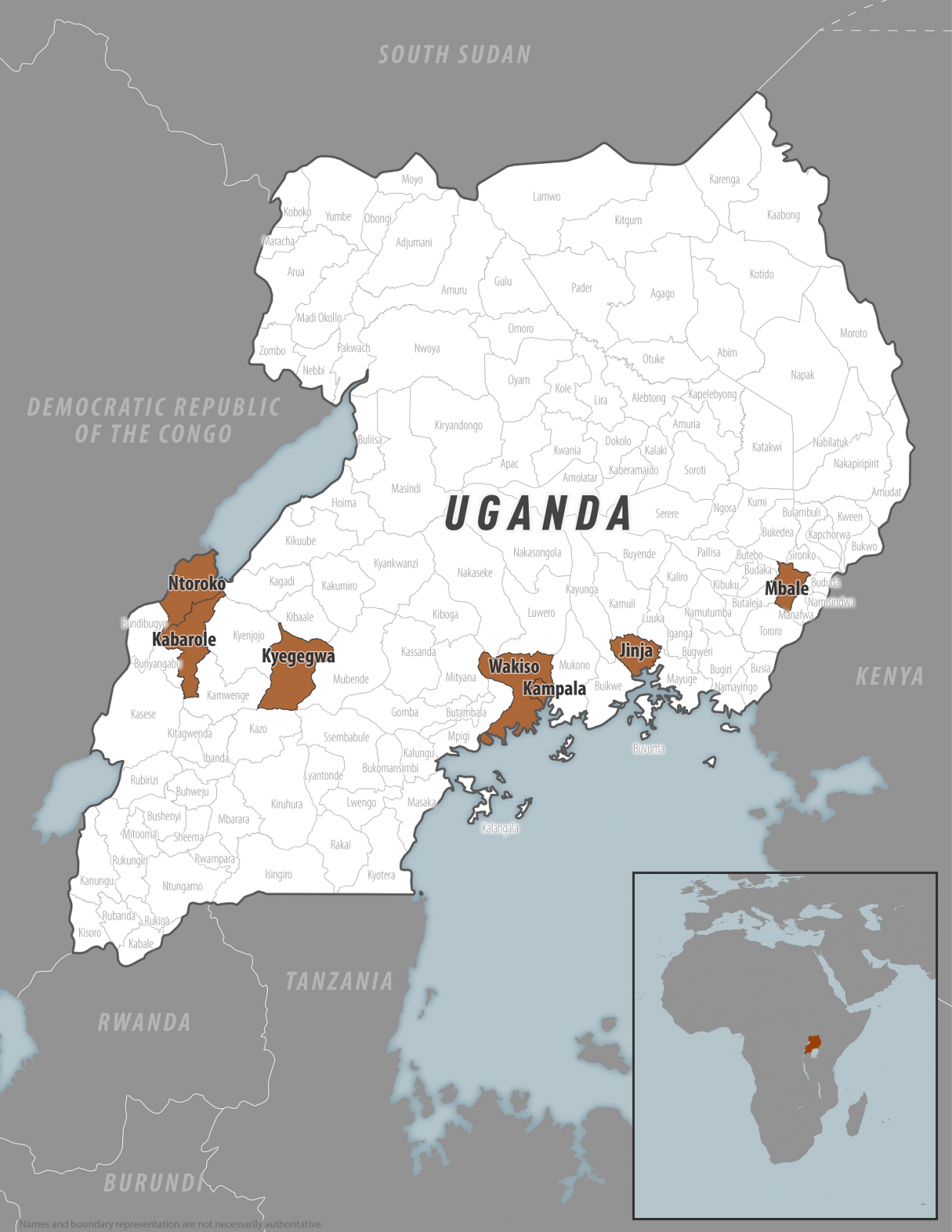
In response to the ongoing Sudan Ebola virus outbreak in the Republic of Uganda, the U.S. government recently updated a Level 2 Travel Health Advisory for this rare and deadly disease.
On March 12, 2025, the Centers for Disease Control and Prevention (CDC) confirmed a Sudan virus disease (SVD) outbreak in the Kampala, Wakiso, Jinja, Mbale, Kyegegwa, Kabarole, and Ntoroko districts.
Since late January 2025, about 14 cases, including four related fatalities, have been reported.
The CDC says local health authorities in Uganda are working to identify infected people and transmission sources, conduct investigations, take action to prevent further transmission, and educate communities and the public about the risks and dangers of SVD.
If you travel to Uganda, the CDC published an extensive list of activities to avoid.
About 200,000 people visited Uganda in 2024. Traveler screening at Ugandan entry points remains active as of March 2025.
Unlike Zaire Ebola, which has approved vaccines and therapeutics, SVD vaccines and therapeutics remain under development as of mid-March 2025.
From a safety perspective, the U.S. Department of State recently updated its Level 3 Travel Advisory to reflect current information.
The State Department advises reconsidering travel to Uganda and exercising increased caution due to potential risks and the unpredictable nature of public demonstrations. The U.S. Embassy in Kampala is available to support U.S. citizens.
Most older adults and immunocompromised individuals are familiar with herpes zoster (HZ), which causes painful rashes upon activating the varicella-zoster virus (VZV).
Although the U.S. FDA has approved a vaccine (Shingrix®) for preventing shingles, its administration is commonly associated with high reactogenicity.
On March 14, 2025, results from a new study published by the journal Nature focused on ten different vaccine candidate designs using two different codon optimizations targeting the VZV glycoprotein E (gE).
For this evaluation, researchers developed several VZV modRNA vaccine candidates targeting the glycoprotein gE, one of the most abundant proteins on the surface of the virion.
A subset of mRNA constructs was formulated into lipid nanoparticles and assessed for their ability to induce specific cellular and humoral immune responses in mice following vaccination.
Notably, the selected mRNA vaccine candidates induced high antibody levels and robust CD4+ and CD8+ immune responses.
Moreover, this study showed that alternate lyophilized vaccines provide comparable immunogenicity to liquid frozen formulations and are stable under long-term storage conditions.
Some of these investigational VZV modRNA candidates, including a lyophilized presentation, are currently being tested in a Phase I/II clinical study sponsored by Pfizer Inc.
This study's primary completion estimate is in late 2025.
While somewhat similar, no herpes simplex virus (HSV) vaccines are approved for use in 2025. However, this is an HSV mRNA vaccine candidate conducting research as of March 2025.