Search API
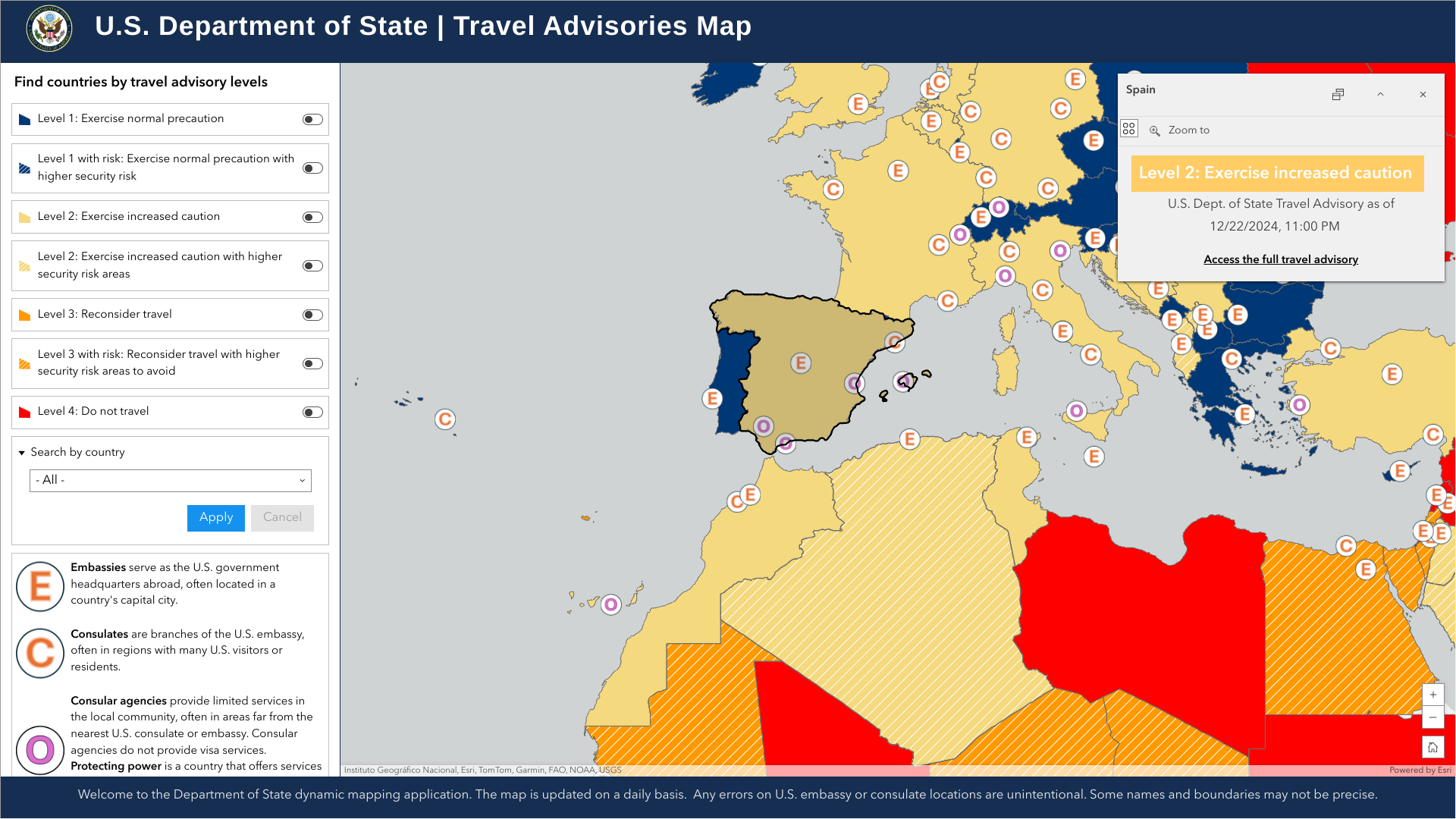
The U.S. Department of State recently reaffirmed its Level 2: Exercise Increased Caution for the Kingdom of Spain.
On December 23, 2024, the State Department stated visitors to Spain should exercise increased due to civil unrest. Furthermore, demonstrations are common and may occur in response to political or economic issues, on politically significant holidays, and during international events.
International travelers visiting Spain should enroll in the Smart Traveler Enrollment Program to receive digital alerts, which makes locating you in an emergency easier.
When in Spain, U.S. citizens can visit the U.S. Embassy at Calle Serrano, 75, 28006 Madrid.
From a health perspective, the U.S. CDC and the UK say visitors should check the list of vaccines and medicines needed at least a month before visiting Spain. For example, the ECDC reported locally acquired dengue cases in Spain in 2024.
Dengue is an Aedes-borne disease established in Spain's Catalonia region.
Colin Johnston, Senior Medical Entomologist at UKHSA, informed local media, "The increasing number of dengue (8) and malaria cases that we are seeing now in the UK are travel-related."
As of December 25, 2024, Dengue is a vaccine-preventable disease.
The Pan American Health Organization (PAHO) recently confirmed that influenza-like illnesses have increased in the North American subregion. Seasonal influenza (flu) became widespread in many sections of the United States in late December 2024.
According to the PAHO and numerical health agencies, most eligible people should get a flu shot that protects against the viruses causing infection.
The PAHO says there are four types of influenza viruses: A, B, C, and D.
As of December 25, 2024, various U.S. FDA-approved flu shots are available at local pharmacies, helping prevent severe influenza infections caused by these viruses.
The good news is pharmaceutical companies are developing vaccines that provide broad-spectrum protection against these every-mutating viruses.
A study published on December 11, 2024, in the journal MDPI, reported in a Phase 2a, double-blind, placebo-controlled study, OVX836, a nucleoprotein (NP)-based candidate vaccine, previously showed a good safety profile, a robust immune response (both humoral and cellular) and a preliminary signal of protection of 84% against confirmed symptomatic influenza after a single intramuscular dose of 180 µg, 300 µg or 480 µg.
Furthermore, T-cell responses were highly cross-reactive against various influenza A strains, both seasonal and highly pathogenic avian strains.
Last month, Osivax announced its ongoing efforts to prepare this vaccine candidate for marketing.
On November 11, 2024, the first participant was vaccinated in a Phase 2a clinical trial (NCT06582277) evaluating OVX836 as a booster in participants vaccinated three to five years ago in earlier Osivax vaccine studies.
The topline results from this trial are expected by the end of 2025.
“This milestone is a significant step forward in our mission to develop a truly broad-spectrum, lasting flu vaccine capable of addressing the ever-evolving threat of influenza. By studying the effects of a booster dose, we aim to deepen our understanding of OVX836’s potential to provide robust and sustained immune protection,” said Dr. Nicola Groth, CMO of Osivax, in a press release.
“Osivax is committed to leveraging innovative science to develop vaccines that protect individuals and help reduce the global healthcare burden associated with seasonal flu epidemics and potential pandemics.”
Osivax is a clinical-stage biopharmaceutical company that aims to develop a pan-respiratory virus vaccine that can prevent all strains of influenza in one shot. The company also intends to expand into other infectious disease indications through combinations and collaborations worldwide.

Despite decades of public health vaccination programs, invasive pneumococcal disease (IPD) continues to cause substantial disease burden, primarily due to Streptococcus pneumoniae serotypes not included in pneumococcal conjugate vaccines (PCV).
Various pharmaceutical companies say next-generation PCVs can extend vaccine coverage of disease-causing serotypes.
To accelerate solutions addressing this health need, Sanofi and SK bioscience announced on December 23, 2024, that they entered into a new collaboration in pneumococcal vaccines with an expanded agreement to develop, license and commercialize next-generation PCVs for pediatric and adult populations.
This expansion builds on the companies’ existing collaboration to develop and commercialize a PCV21 pediatric vaccine, for which the phase 3 clinical program commenced last week. This vaccine candidate is the first-ever PCV containing more than 20 serotypes to enter a phase 3 clinical study in infants and toddlers.
The PCV21 phase 3 program, based on positive phase 2 results communicated in 2023, will include infants, toddlers, young children, and adolescents across multiple geographies, including the US, Europe, Australia, Asia, and Latin America.
Thomas Triomphe, Executive Vice President of Vaccines at Sanofi, commented in a press release on December 23, 2024, “Given the vast unmet public health needs in IPD, we’re delighted to expand this collaboration and continue our pursuit of innovative work in PCV. Our collaboration leverages SK bioscience’s capabilities and Sanofi’s expertise in developing and bringing innovative vaccines to people worldwide to reduce the global impact of pneumococcal disease.”
The World Health Organization says pneumococcal vaccinations continue to prevent pneumococcal disease. While vaccines can not prevent every kind of community-acquired pneumonia, they work against the most common bacteria types.

The Galveston County Health District recently informed the Texas Gulf Island community about a Hitchcock High School staff member who has been diagnosed with active tuberculosis (TB).
On December 20, 2024, the District confirmed the patient received treatment at a local hospital. It is actively investigating the situation and has contacted 120 students and staff who may have been exposed to the infectious bacterial disease to arrange evaluations and testing.
Free screening is offered at the Galveston County Health DistrDistrict'sunicable Disease Office by calling (409) 938-2354.
The District wrote in a media release, 'We understand this situation may be concerning, and we want to reassure the community that we are taking all necessary precautions.'
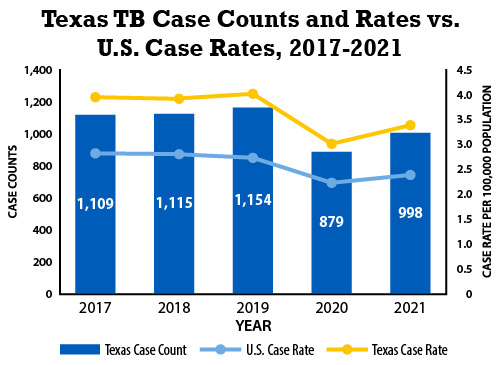
Over the past few years, the state of Texas, like the entire United States, has reported an increase in TB cases.
Texas reported 1,235 TB cases in 2023, compared to 1,100 cases in 2022.
In 2023, Galveston County reported 11 TB cases, while fifty miles north in Harris County, 269 TB cases were confirmed last year.
The U.S. CDC reports a 16% increase in TB cases nationwide in 2023 compared to 2022.
While TB is a vaccine-preventable disease, the 100-year-old BCG vaccine is about 50% effective and is generally offered at public health departments. However, several enhanced BCG vaccine candidates are conducting late-stage clinical trials, with aspirations for approval in 2025.
With over 1.1. billion doses already delivered, the Global Health Technologies Coalition (GHTC) recently honored the novel oral polio vaccine type 2 (nOPV2) development consortium with its 2024 Innovating for Impact Award.
The GHTC awards, issued annually in December, recognize multisectoral partnerships and policymakers helping to transform breakthrough scientific research into lifesaving drugs, diagnostics, vaccines, and other health tools for unmet global health challenges.
Dr. Ananda Bandyopadhyay, Deputy Director of Technology, Research, and Analytics, Polio Team, Bill & Melinda Gates Foundation, commented in an announcement, "The fight against polio has always been a story of partnerships…So many countries, partners, and people came together to develop this vaccine. This nOPV2 journey is an example of pushing the boundaries of innovation and doing it as a global team."
nOPV2 is derived from the live, infectious virus and has been 'triple-locked' using genetic engineering to prevent it from producing mutations and causing paralysis. As a result, nOPV2 is reported to be more genetically stable than previous oral polio vaccines.
The WHO's SAGE recently recommended that, where feasible, the concomitant use of Inactivated polio vaccine (IPV) and nOPV2 be used for initial poliovirus outbreak response vaccination campaigns.
The IPV has been offered in the U.S. since 2000, while the nOPV2 has been provided in Africa in 2024.
Polio is a very contagious infectious disease that can lead to permanent paralysis. Worldwide polio cases have dropped by 99% since 1988 thanks to global vaccination efforts.




