Search API
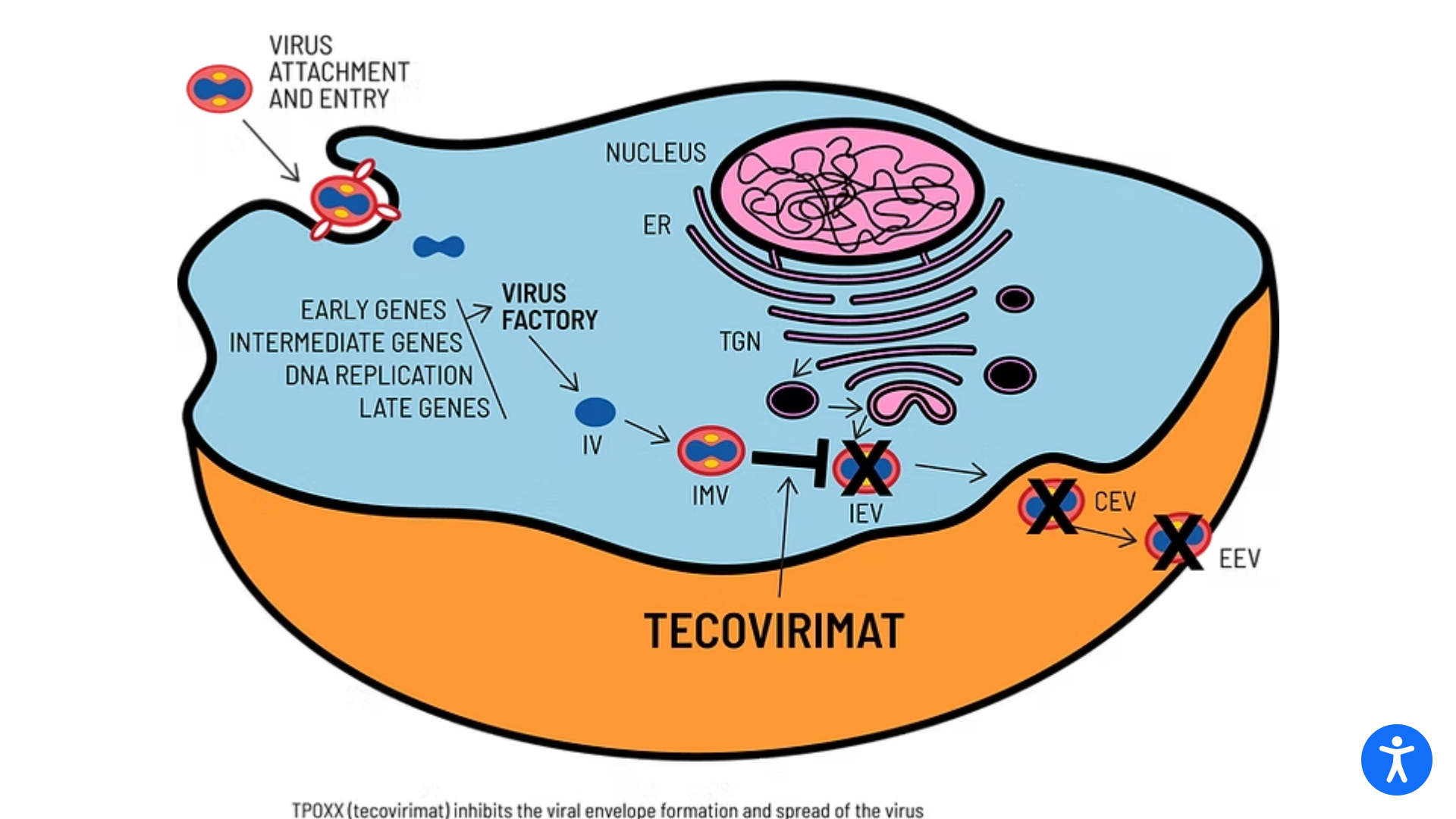
SIGA Technologies announced that its antiviral treatment TEPOXX (tecovirimat 200 mg capsules, TPOXX) had received regulatory approval in Japan for the treatment of smallpox, mpox, cowpox, as well as complications following smallpox vaccination in adults and pediatric patients weighing at least 13 kg. TEPOXX is the first antiviral therapy approved for the treatment of orthopoxviruses.
As of January 2, 2025, SIGA has delivered an order of TEPOXX to help build Japan’s strategic national stockpile.
TEPOXX is a highly targeted small-molecule antiviral that inhibits the VP37 protein found on the surface of all orthopoxviruses. By preventing the virus from exiting infected cells, TEPOXX slows the spread of the infection, enabling the immune system to clear the virus.
TPOXX is approved in the U.S. and Canada for the treatment of smallpox. In the European Union and the United Kingdom, marketed as Tecovirimat-SIGA, it is approved for treating smallpox, mpox, and cowpox and to treat complications following smallpox vaccination.
The Japanese approval is based on data from 15 clinical trials of oral TEPOXX in over 800 healthy volunteers, including a pivotal repeat-dose phase 1 pharmacokinetics (PK) trial involving 20 healthy volunteers conducted in Japan. TEPOXX has also been studied in NHPs infected with the variola virus, which causes smallpox, where TEPOXX demonstrated improved survival and reduced lesions.
In 2024, the U.S. NIH announced unsatisfactory results regarding mpox usage.
In 2024, the unfortunate leader in extensively drug-resistant (XDR) Shigellosis outbreaks was the state of California, with 4,365 of the 20,621 cases reported nationwide.
A Brief Report published by AJIC on December 9, 2024, describes a mutation of Shigella sonnei, a strain of Shigella bacteria resistant to five of the antibiotic classes most commonly prescribed for such infections.
This report describes a distinct genetic mutation that made the bacteria resistant to another class of antibiotics, the cephalosporins.
These researchers say this XDR Shigella strain appears unique to LA country.
"These cases highlight the rapid expansion of extensively drug-resistant Shigella in the United States and the urgent need for appropriate detection and management,' wrote these UCLA and Quest researchers.
Since first detected in California in 2017, XDR Shigella has been increasing in prevalence. By 2022, 3.2% of Shigella isolates were XDR. From January through May 2024, 12% (118/978) of California Shigella isolates were reported as XDR. Shigella sonnei isolates accounted for the most significant percentage (78%) of cases.
The U.S. CDC estimates that Shigellosis cases occur annually, making it the third most common bacterial enteric disease. As of week #52, ending December 28, 2024, the CDC confirmed 20,621 Shigella cases, led by California and New York (2,990).
In 2023, the CDC reported 17,176 cases in the U.S.
From a prevention perspective, Valneva SE and LimmaTech Biologics AG are co-developing the Shigella4V (S4V), a tetravalent bioconjugate vaccine candidate against Shigellosis. On November 13, 2024, the companies launched a Phase 2b clinical trial for Shigella4V.
Previously, the U.S. FDA granted Shigella4V Fast Track designation.

The U.S. Department of Health and Human Services (HHS) today announced that it is awarding $306 million in additional funding to support its response to H5N1 avian influenza outbreak in annimals.
The HHS Secretary stated on January 3, 2025, that although the risk to humans remains low, federal officials are preparing for various outbreak scenarios.
"Preparedness is the key to keeping Americans healthy and our country safe. We will continue to ensure our response is strong, well-equipped, and ready for whatever is needed."
About $183 million of this funding is earmarked for regional, state, and local preparedness programs, such as hospital readiness and boosting emerging-pathogen training and treatment, focusing on avian flu activities.
Previously, HHS funded the development of various avian influenza vaccines. For example, in 2020, the U.S. FDA approved the CSL Seqirus Inc. Audenz™ monovalent, adjuvanted, cell-based inactivated subunit vaccine.
These vaccines are not commercially available in January 2025.
In June 2024, the WHO published the Pandemic Influenza Pandemic Framework's partnership Contribution High-Level Implementation Plan III, which outlines the strategy for strengthening global pandemic influenza preparedness from 2024 to 2030.

India has recently increased in popularity as a destination for United States travelers. Last year, the U.S. National Travel and Tourism Office tanked India as the second most popular international travelers destination.
While tourism has increased, so has the number of Chikungunya virus outbreaks in India.
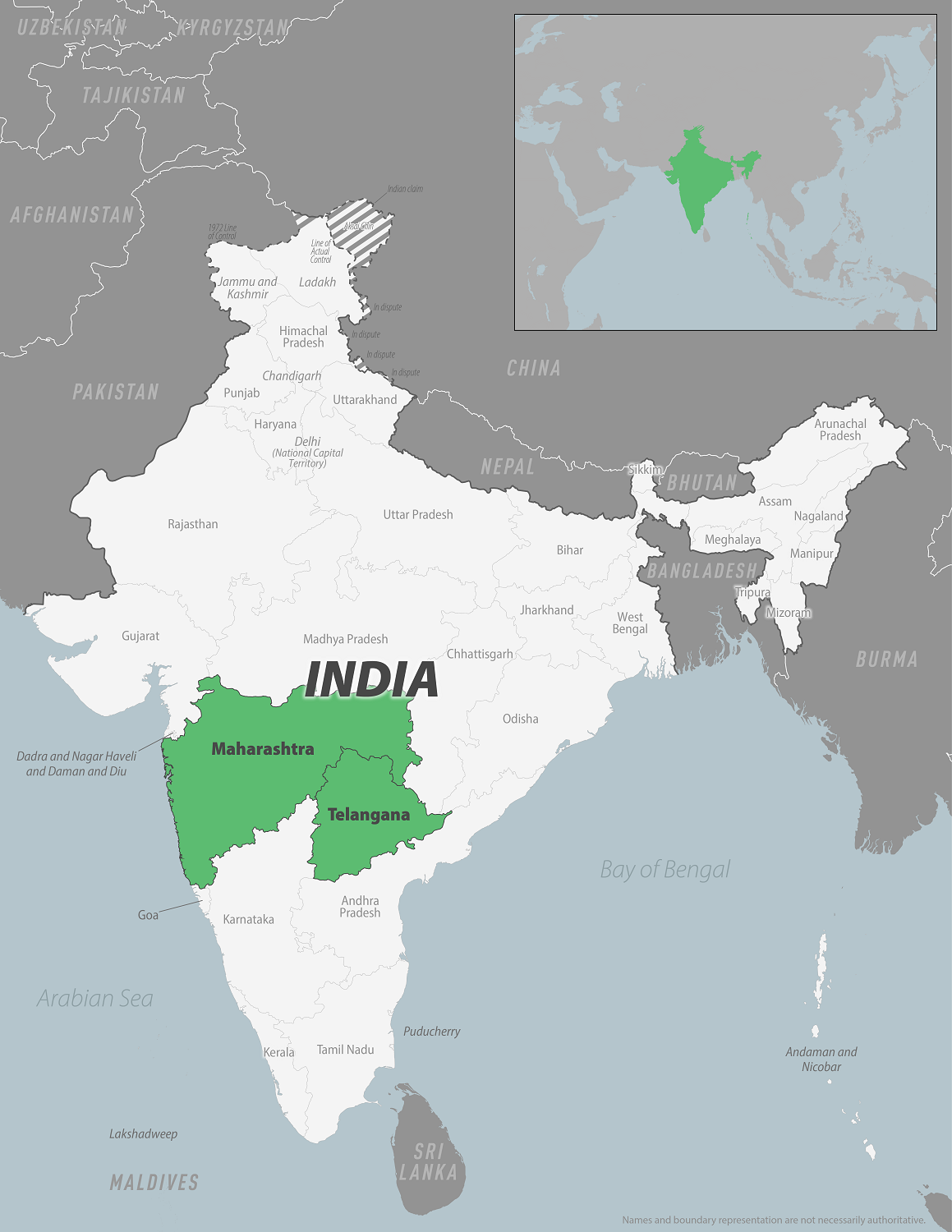
On December 19, 2024, the U.S. CDC reported an outbreak of Chikungunya in India's Maharashtra and Telangana states.
According to India's National Center for Vector-Borne Diseases Control, 192,343 CHIKV cases were reported in 2024. Those infected with this mosquito-transmitted disease develop some symptoms and many report long-term symptoms.
Pune's National Institute of Virology has indicated that Chikungunya virus variants are causing more cases and lingering symptoms in 2024.
A study published on October 19, 2024, determined that the incidence of post-chikungunya chronic rheumatism and fatigue and the impact on quality of life and chronic fatigue in adults seven years after infections were significant.
These experts have identified Chikungunya's "Indian Ocean lineage," first identified in 2006, as the primary cause of the recent surge in cases and lingering symptoms. This novel lineage has also spread globally.
"India's ongoing Chikungunya outbreak is a good reminder for international travelers about this often forgotten mosquito illness and the importance of vaccination. Infection can lead to long-term health issues such as chronic joint pain, and people 65 years and older are especially at risk for complications," Jeri Beales, MSN, RN, informed Vax-Before-Travel.
"The approved vaccine is given as a single dose, and studies show it is highly effective for at least several years after receiving. The U.S. CDC does not recommend every traveler to India get the vaccine. It is only approved for people 18 years and older in the U.S., so be sure to talk to your local travel clinic or physician," added Beales, who leads Destination Health Clinic, a Boston, Massachusets area travel health provider specializing in health education and vaccination for international travelers.
Since 2013, the Asian genotype has led to Chikungyna outbreaks in the Region of the Americas, which will continue in 2026.
Last year, the Pan American Health Organization reported over 421,018 Chikungunya cases and 211 related deaths in the Americas.
The CDC's Level 2—Practice Enhanced Precautions Travel Health Advisory recommends the FDA-approved Chikungunya vaccine for certain visitors and long-term residents in India and other areas reporting outbreaks.
As of January 2025, Valneva SE's IXCHIQ® (VLA1553) vaccine is offered at many travel clinics and pharmacies in the U.S.
In the Region of the Americas, 2024 has been marked as the worst year ever for dengue virus infections, 12.8 million, and related fatalities, 7,855.
From a country perspective, the United States has experienced a significant impact, with dengue cases increasing by approximately 43%. According to the U.S. CDC, 8,863 dengue cases were reported as of mid-December 2024, compared to 6,164 cases in 2023.
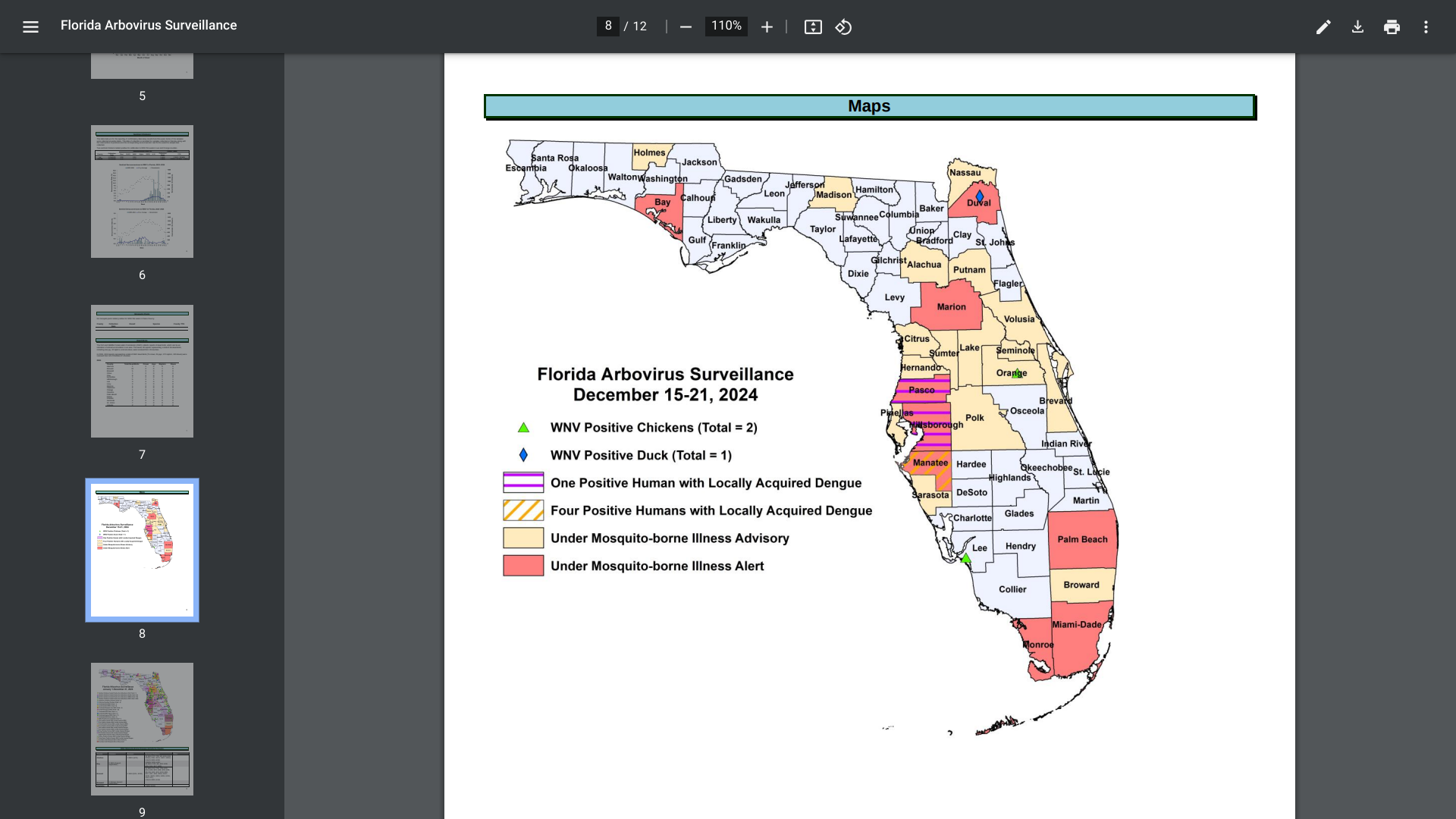
Within the U.S., the Florida Department of Health recently reported some unsettling news.
As of late December 2024, 911 travel-associated dengue cases had been reported, mainly by visitors to Brazil (61), Cuba (506), and Puerto Rico (41). Additionally, 85 locally acquired dengue cases were reported from ten counties, led by Miami-Dade (47).
In 2023, 609 travel-associated dengue cases were reported, and 186 humans contracted dengue while in Florida.
With international travel expected to increase in 2025, this CDC data suggests that more international visitors may unintentionally introduce one of the four dengue viruses in Florida next year.
Furthermore, since mosquitoes transmit dengue, locally acquired infections will likely increase next year.
From a disease prevention perspective, there is hope that access to dengue vaccines may improve in 2025 as approved vaccines increase production and vaccine candidates seek approvals.
The Costa Rican Ministry of Health published a notice in December 2024 regarding changes to yellow fever certificate requirements for travelers, effective January 11, 2025.
The countries that are now considered at risk and for which yellow fever vaccination against yellow fever is required are the following: Bolivia, Brazil, Colombia, Ecuador, Guyana, French Guiana, Paraguay, Peru, Suriname, Venezuela, and Trinidad and Tobago. Additionally, countries in Africa have been added to Costa Rica's list.
When visiting Costa Rica, a yellow fever vaccination (YF-VAX or Stamaril) certificate may be required at the airport. Foreigners who arrive in Costa Rica and do not present the International Certificate of Vaccination against Yellow Fever to the immigration authority will not be allowed to enter the territory.
As of December 31, 2024, the YF vaccine is not part of the mandatory vaccination schedule, so it must be purchased at private pharmacies.
In addition to YF, Costa Rica has reported Chikungunya (45), Dengue, and Zika (25) cases in 2024.
Most of this year's Dengue cases (30,000) have been detected near San Jose in the mountains, not at Costa Rica's beaches.
Previously, in early December 2024, the U.S. State Department issued a Level 2: Exercise Increased Caution, Travel Advisory, for the Republic of Costa Rica. This U.S. advisory says visitors to Costa Rica should exercise increased caution due to various civil unrest targeting tourists.
Last year, about 2.4 million people visited Costa Rica, many traveling from the United States.