Search API
Merck announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) for active immunization for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.
Pneumococcal disease is an infection caused by Streptococcus pneumoniae. There are about 100 types of pneumococcal bacteria, and they can affect adults differently than children.
“Invasive pneumococcal disease and pneumococcal pneumonia remain critical public health challenges worldwide,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on January 31, 2025.
The CHMP’s recommendation for marketing authorization in the European Union (EU), Iceland, Liechtenstein, and Norway will now be reviewed by the European Commission. A final decision is expected by the second quarter of 2025.
If approved in the EU, it would mark the fourth authorization of CAPVAXIVE for preventing invasive pneumococcal disease and pneumococcal pneumonia in adults.
CAPVAXIVE was first approved in the U.S. in June 2024, Canada in July 2024, and Australia in January 2025. It is being reviewed in Japan, and other worldwide regulatory filings are underway.
In the U.S., pneumococcal vaccines are recommended for most people and are available at most community pharmacies. These vaccines may not work for everyone.
During 2024, the United States reported several measles outbreaks primarily related to unvaccinated international travelers. According to new reports, the State of Texas may lead this unfortunate list in 2025.
Today, the Texas Department of State Health Services (DSHS) announced two confirmed measles cases in Gaines County residents, located southwest of Lubbock, Texas. Both instances involve unvaccinated school-age children who were hospitalized in Lubbock.
As of January 30, 2025, these children have been discharged.
These newly identified cases are in addition to two confirmed measles cases reported in Harris County in 2025.
The Houston Health Department (HHD) identified two confirmed measles cases associated with international travel. Both adults were unvaccinated against measles.
HDD says anyone exposed to measles should monitor themselves for symptoms, including a rash, high fever, cough, runny nose, and red, watery eyes. Symptoms can appear 7 to 21 days after exposure. If you show symptoms of measles, call your healthcare provider to make arrangements for evaluation and treatment.
On January 23, 2025, HHD stated, 'Due to the highly contagious nature of this disease, additional (measles) cases may occur.'
Houston and Harris County are home to about 5 million people and are gateway cities with two international airports.
Crockett Tidwell RPh, CDCES, CTH, informed Vax-Before-Travel News, "Measles is extremely contagious; nine9 out 10 people in the same room will become infected if they do not have immunity."
"All it takes is one international traveler to infect every vulnerable person they come in contact with when they come home," added Tidwell, Clinical Services Manager, International Society of Travel Medicine Certificate in Travel Health™.
To alert travelers of the global measles risk, the U.S. CDC recently updated a Travel Health Advisory, which identified 59 countries reported measles cases. The CDC recommends people speak with a travel vaccine expert about immunization options before visiting these countries in 2025.

Ocean Biomedical recently announced that its Scientific Co-founder, Dr. Jonathan Kurtis, MD, PhD, and his research team have received additional funding from the U.S. National Institutes of Health (NIH) to advance their malaria vaccine research.
With the support of a $4.6 million non-governmental Foundation grant, Dr. Kurtis’ team is now testing three vaccine candidates in non-human primates. These candidates aim to block the malaria parasite’s ability to enter and exit red blood cells.
The research also explores the feasibility of using lipid-encapsulated messenger ribonucleic acid (mRNA) technology as a delivery mechanism.
In December 2024, Dr. Kurtis secured a $3.5 million NIH grant to identify vaccine targets further to protect against severe malaria in children.
Malaria remains a devastating global health challenge, claiming the lives of over 500,000 children annually in sub-Saharan Africa.
As of January 30, 2025, two malaria vaccines are available in Africa. However, they are not available in the U.S.
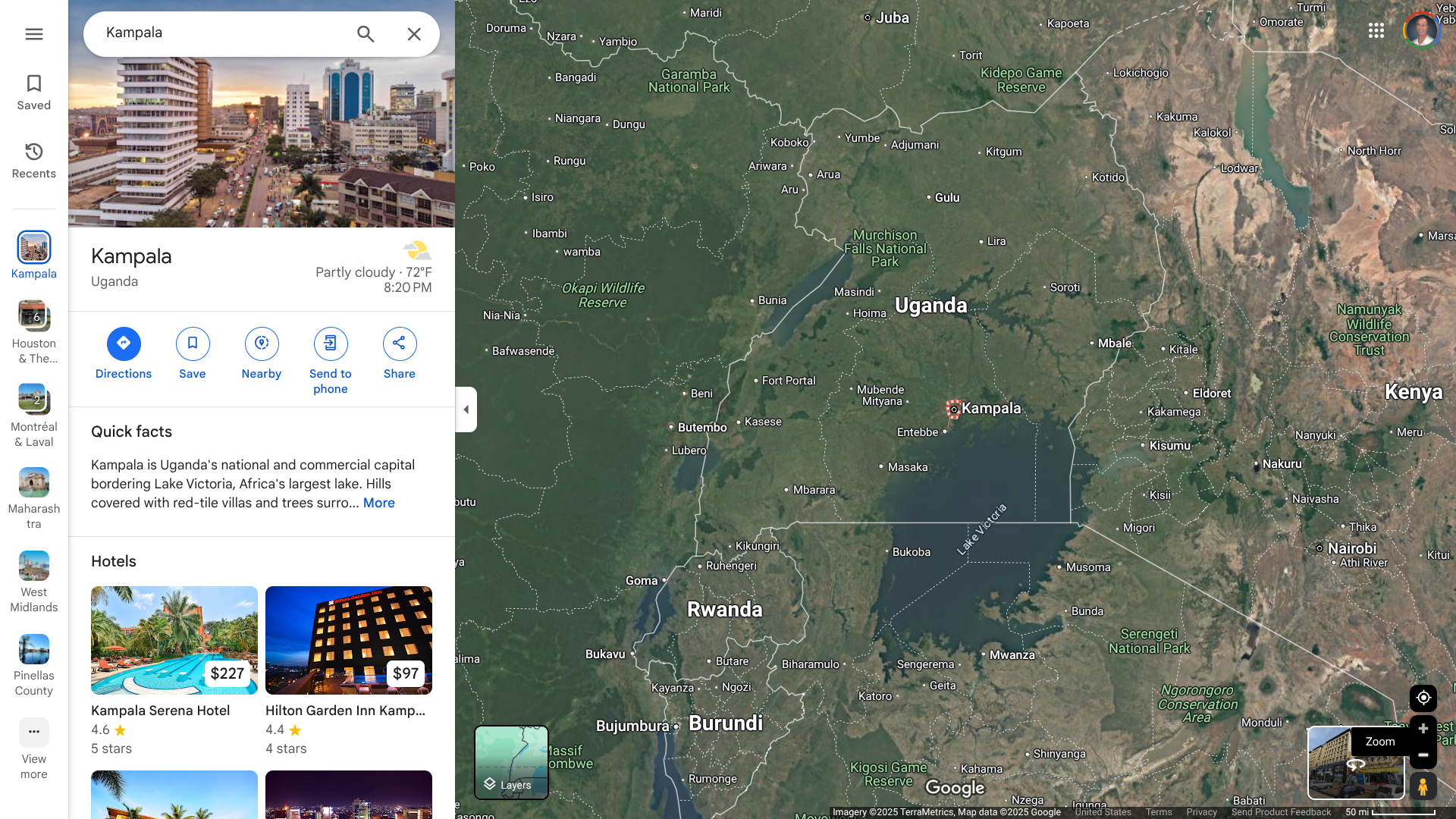
Following the confirmation of an outbreak of Sudan virus disease in the Republic of Uganda, the World Health Organization (WHO) announced it is mobilizing efforts to support the national health authorities in containing a potential outbreak in Kampala.
The identification of the case in a densely populated urban requires a rapid and intense response, says the WHO.
As of January 30, 2025, a nurse from Mulago National Referral Hospital in the capital, Kampala, a city with about 1.8 million residents, has been reported with this disease.
A total of 45 contacts, including health workers and family members of the confirmed case (deceased), have been identified and are currently under close monitoring. No other health workers or patients have shown symptoms of the disease.
“We welcome the prompt declaration of this outbreak, and as a comprehensive response is being established, we are supporting the government and partners to scale up measures to quickly identify cases, isolate and provide care, curb the spread of the virus, and protect the population,” said Dr Matshidiso Moeti, WHO Regional Director for Africa, in a press release.
Eight previous outbreaks of the Sudan virus disease have occurred, five in Uganda and three in Sudan. Uganda last reported an outbreak in 2022.
Sudan virus disease is a severe, often fatal illness affecting humans and other primates. It is caused by Orthoebolavirus Sudanese (Sudan virus), a viral species belonging to the same genus as the virus that causes Ebola virus disease.
Case fatality rates of Sudan virus disease have varied from 41% to 100% in past outbreaks.
While no licensed vaccines for the Sudan virus disease exist, the WHO coordinates with developers to deploy candidate vaccines and other public health measures.
The WHO stated that experimental vaccines would be deployed once all administrative and regulatory approvals were obtained.
GSK plc and the University of Oxford (Oxford) today announced that they have entered a new research collaboration focused on the potential of cancer prevention through vaccination.
GSK will invest up to £50 million ($62m) over three years to support this early research.
Confirmed on January 27, 2025, the GSK-Oxford Cancer Immuno-Prevention Programme will conduct translational research, exploring precancer biology to generate key insights on how cancer develops in humans that could inform new approaches to cancer vaccination.
Professor Irene Tracey, Vice-Chancellor of the University of Oxford, commented in a press release, “This partnership represents a step forward in cancer research. By working with GSK to unite experts in clinical trials, immuno-oncology, vaccinology, and precancer research from across the University of Oxford, we aim to unlock the potential of cancer vaccines and bring hope to patients worldwide.”

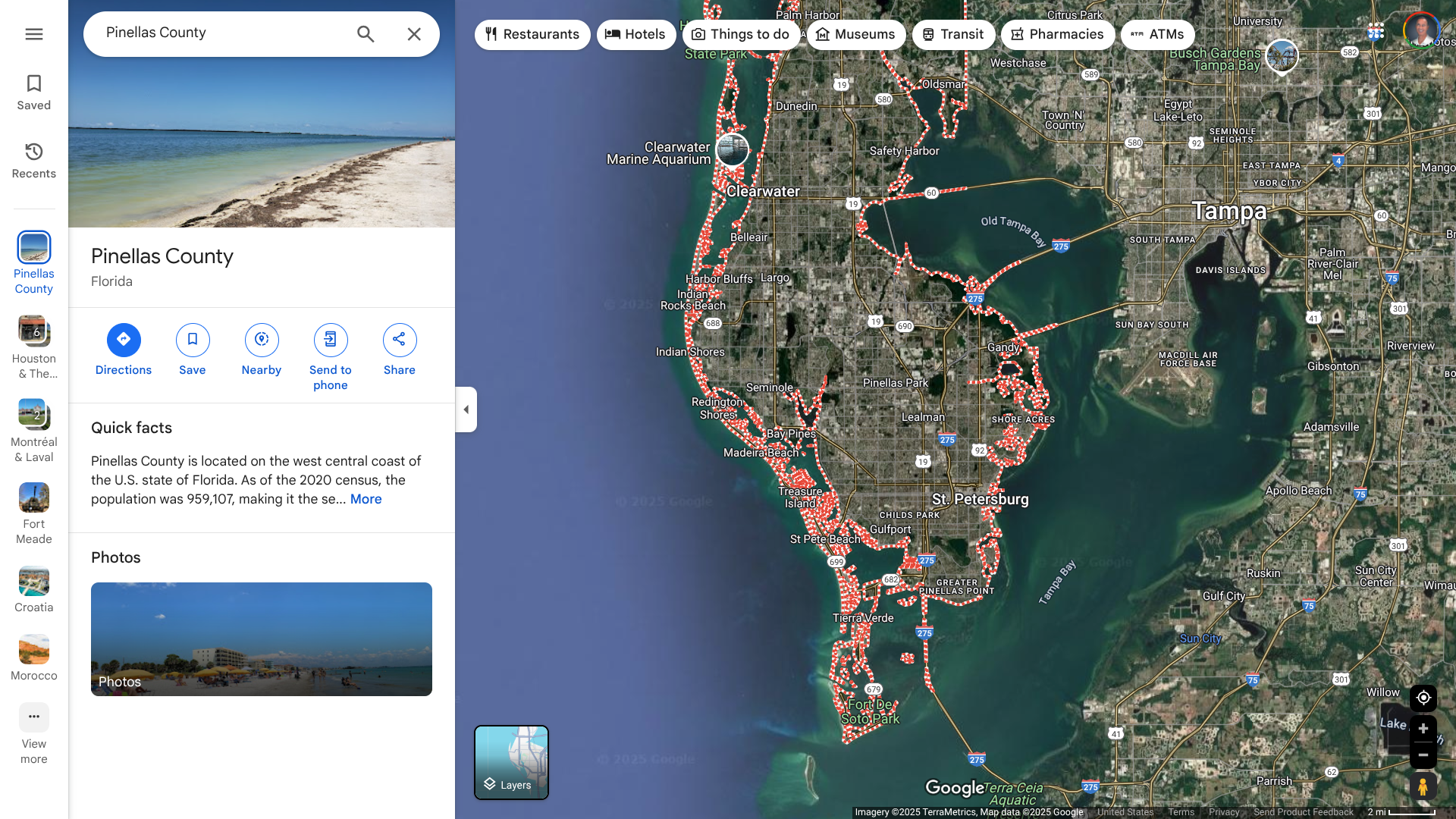
The Florida Health Department recently confirmed a record number of Vibrio vulnificus (V. vibrio) cases and deaths in 2024. While infections are rare, this increase was related to hurricanes in the year's second half.
As of January 3, 2025, Florida reported 83 V. vibrio cases and 18 deaths last year. Pinellas County (Tampa Bay) led all counties with 15 cases and three deaths.
In 2023, there were 46 cases and 11 related deaths.
About 80,000 cases of vibriosis happen each year in the United States.
Vibrio is a naturally occurring bacterium in warm seawater. Brackish water is a mixture of salt and fresh water often found where rivers meet the ocean. People can get vibriosis after swallowing Vibrio or getting it in a wound.
Florida and the U.S. CDC recommend not to enter the salt water if you have fresh cuts or scrapes.
Furthermore, there are no protective V. vibrio vaccines available in 2025.
However, various travel vaccines are available to protect people when visiting Florida.
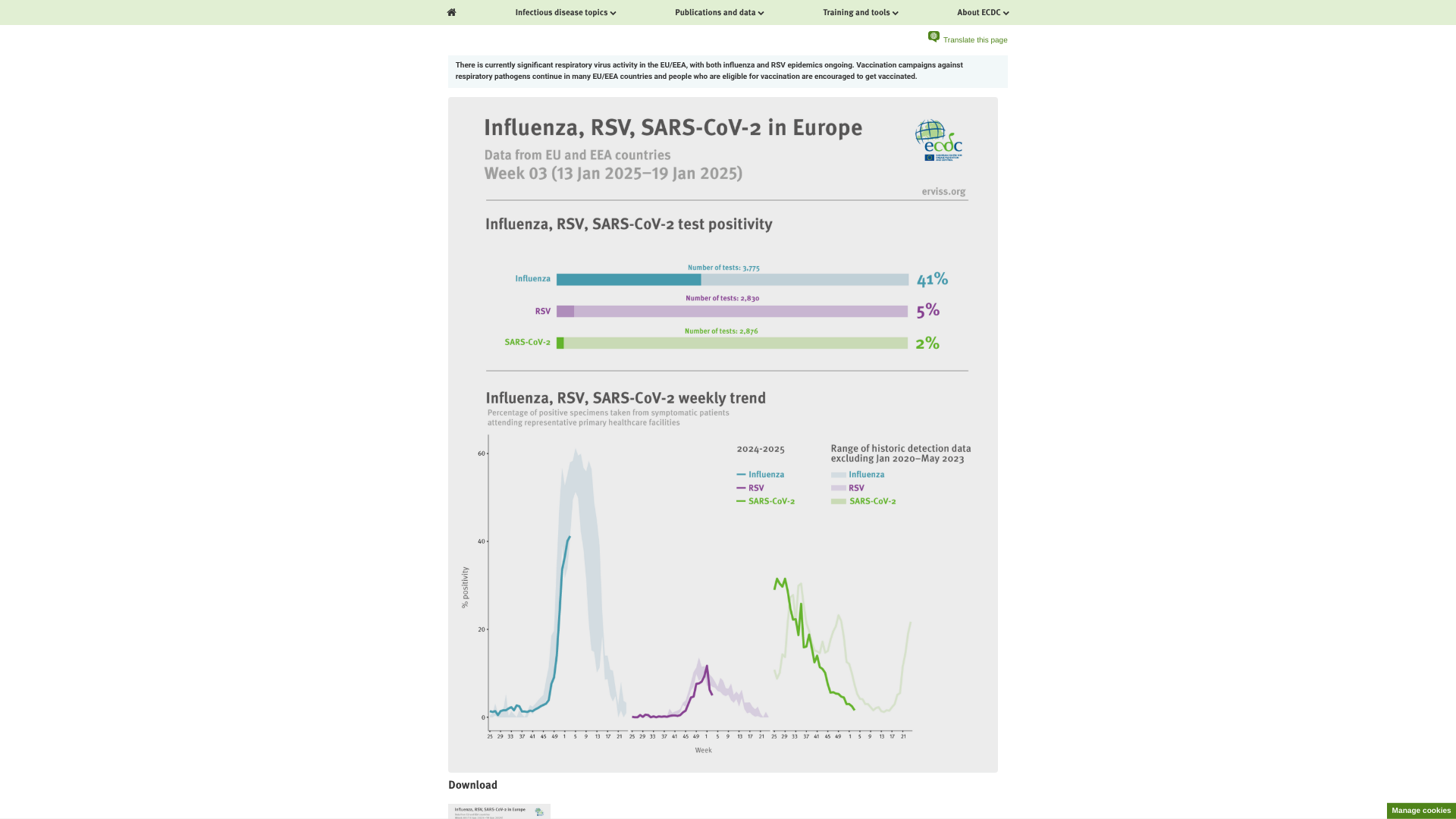
The European Centre for Disease Prevention and Control (ECDC) recently published recommended actions for response regarding respiratory viruses and preventive vaccines during the 2025 winter season.
On January 24, 2025, the ECDC stated that primary and secondary care consultation rates have increased in several countries recently and that significant respiratory virus activity is occurring in Europe.
Seasonal influenza and respiratory syncytial virus (RSV) epidemics are ongoing.
All indicators point to widespread high influenza activity in the EU/EEA, albeit some countries are now observing slightly decreasing trends in test positivity following a peak in transmission.
In recent weeks, RSV activity decreased overall at the EU/EEA level, although the country-level picture remains mixed.
The ECDC says 'vaccination is the most effective measure to protect against more severe forms of viral respiratory diseases. Those eligible for vaccination, particularly those at higher risk of severe outcomes, are encouraged to get vaccinated.'
According to Stattista, Europe accounts for over half of international tourist arrivals worldwide, with inbound arrivals exceeding 700 million in 2023.
As of January 26, 2025, the U.S. CDC and the ECDC encourage international travelers to be updated on travel vaccines relevant to their pending trip abroad.