Search API
The journal NPJ Vaccines recently reported that researchers have developed and characterized a novel dual-target single-shot vaccine candidate that protects against Ebola (EBOV) and Yellow Fever (YFV) infection.
Announced on July 11, 2023, the YF-EBO pre-clinical vaccine candidate could help communities combat simultaneous EBOV and YFV epidemics, such as in Africa.
While there are approved vaccines for Ebola (Ervebo®) and Yellow fever (Stamaril®) in 2023, a single combo vaccine could enhance vaccination campaigns.
EBOV is a member of the Filoviridae family that causes severe and acute systemic disease in humans, known as Ebola virus disease, with mortality rates up to 80%.
YFV is a mosquito-borne flavivirus causing severe hemorrhagic disease in humans.
Yellow fever is endemic in Central and South America, as well as sub-Saharan Africa, where EVD surges.
Despite the availability of a very efficient live-attenuated yellow fever vaccine (Stamaril), annually, an estimated 51,000–380,000 severe cases of YF still occur, resulting in 19,000–180,000 deaths.
The re-emergence of YF outbreaks can be mainly attributed to low vaccine coverage due to supply issues.
Therefore, alike for EVD, a second-generation YFV vaccine with a sustainable supply could deliver measurable benefits during dual outbreaks.
Future studies must address whether this observed cross-reactive humoral immunity is sufficient to also provide cross-protection against heterologous challenge, ideally in step-up models using original filoviruses under BSL4 conditions, wrote these researchers.

IAVI announced today that the initial participants had been vaccinated with a Sudan virus (SUDV) vaccine candidate in a first-in-human Phase I clinical trial in the U.S.
As of June 27, 2023, the IAVI C108 IAVI-sponsored trial is funded by the Biomedical Advanced Research and Development Authority (BARDA).
IAVI C108 will occur at two U.S.-based clinical trial sites, where the vaccine candidate will be administered intramuscularly at three dosage levels.
This is essential news since there are no SUDV vaccines available.
Furthermore, like the Zaire Ebolavirus (ZEBOV), SUDV is responsible for recurring viral hemorrhagic fever outbreaks across sub-Saharan Africa.
In past Ebola outbreaks, the estimated case fatality ratios of SUDV disease have varied from 41% to 100%.
This study evaluates the safety and immunogenicity of an investigational SUDV vaccine candidate previously donated to IAVI by Merck. This investigational SUDV vaccine candidate was produced for IAVI from an existing investigational bulk drug substance previously manufactured by Merck.
IAVI is responsible for all aspects of the candidate’s future development, including demonstrating equivalence between this SUDV vaccine candidate and IAVI’s other SUDV vaccine candidate, which utilizes the same viral vector but is manufactured using a new production platform.
The SUDV vaccine candidate being evaluated in IAVI C108 uses the same recombinant vesicular stomatitis virus (rVSV) viral vector platform as ERVEBO®, Merck’s single-dose ZEBOV vaccine, which is licensed in the U.S., U.K., European Union, Canada, Switzerland, and 10 African countries.
“IAVI C108 represents an important first step toward generating the data needed for eventual licensure of an rVSV-SUDV vaccine. The development and licensure of ERVEBO® have resulted in an important tool in Ebola Zaire outbreak responses. If proven effective, we’re hopeful that a vaccine candidate built on the same viral platform will be similarly important in future SUDV outbreaks,” said Swati Gupta, Ph.D., vice president and head of emerging infectious diseases and epidemiology at IAVI, in a related press release.
The rVSV platform has been used extensively in adults and children. The underlying vesicular stomatitis virus is a common animal virus that does not cause serious illness in humans and has been investigated extensively as a vaccine vector.
In the vaccine platform, it is engineered to encode a surface protein from a target pathogen, in this case, SUDV, to prompt the body to mount an immune response.
Much of the research and development on IAVI’s rVSV platform is performed at the IAVI Vaccine Design and Development Lab in Brooklyn, New York.

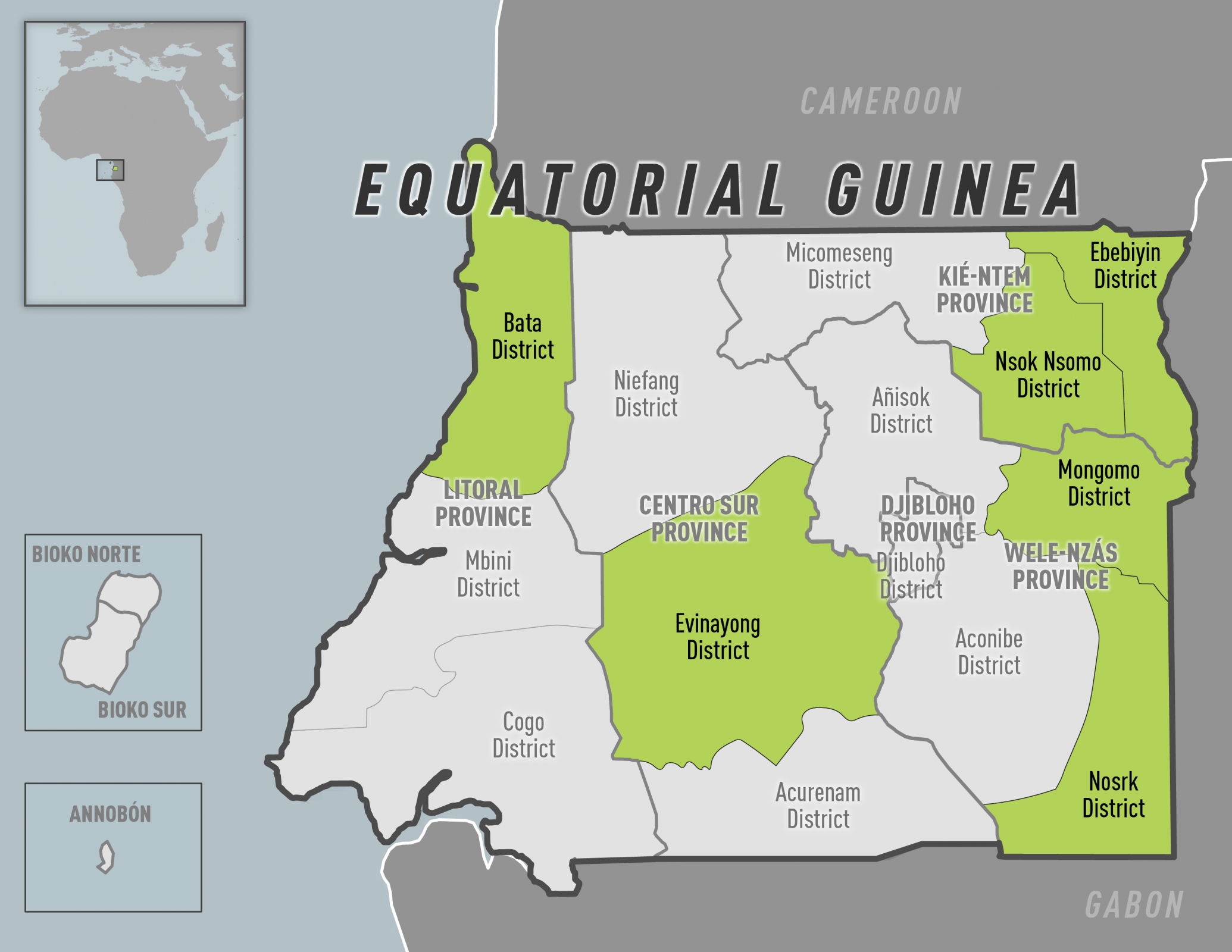
The outbreak of Marburg Virus Disease in Equatorial Guinea ended today with no new cases reported over the past 42 days after the last patient was discharged from treatment.
The outbreak, declared on February 13, was the first in Equatorial Guinea.
A total of 17 laboratory-confirmed cases and 12 deaths were recorded.
Five districts in four of Equatorial Guinea’s eight provinces were affected.
The western Litoral province Bata district was worst-hit, with 11 laboratory-confirmed cases reported. Among the reported cases, many were closely linked either through social gatherings and networks or geographically.
“While outbreak-prone diseases continue to pose a major health threat in Africa, we can bank on the region’s growing expertise in health emergency response to act quickly and decisively to safeguard the health and avert widespread loss of life,” said Dr. Matshidiso Moeti, World Health Organization (WHO) Regional Director for Africa, in a press release on June 8, 2023.
“The hard work by Equatorial Guinea’s health workers and support by partner organizations has been crucial in ending this outbreak. WHO continues to work with countries to improve measures to detect and respond effectively to disease outbreaks,” Dr Moeti added.
To support Equatorial Guinea’s response to the just-ended outbreak, WHO deployed experts in epidemiology, clinical management, health operations, logistics, risk communications, and infection prevention and control.
The Organization worked with the health authorities to set up a treatment center, provided medical supplies, including antivirals, and trained health workers in the critical aspects of outbreak control.
The WHO also supported the efforts by the authorities in neighboring Cameroon and Gabon to ramp up outbreak readiness and response.
Although the outbreak has ended, WHO continues to work with Equatorial Guinea to maintain measures such as surveillance and testing to enable prompt action should flare-ups of the virus occur, with the training provided during the outbreak helping to strengthen readiness capacity.
Marburg is in the same family as the virus that causes Ebola Virus Disease.
The Marburg virus is transmitted to people from fruit bats and spreads among humans through direct contact with the bodily fluids of infected people, surfaces, and materials.
In Africa, the first outbreak of Marburg was recorded in South Africa in 1975, followed by two others in Kenya in the 1980s. Since then, outbreaks have been reported in Angola, the Democratic Republic of the Congo, Ghana, Guinea, Uganda, and most recently, Equatorial Guinea and Tanzania.
Local media recently reported a case of Ebola virus disease in The Democratic Republic of Congo (DRC) on May 8, 2023. The last Ebola outbreak in the DRC occurred in 2022.
“Regarding Ebola virus disease surveillance, we received a sample that turned out to be positive from the Kyondo health zone and in the Butembo site. We have received four samples, and among the four, one is positive,” commented Damulo Luhavo, the communicator of the provincial health division of Butembo.
Luhavo called on the local population to continue to be vaccinated.
As of May 13, 2023, various Ebola vaccines and Monoclonal Antibody therapies are available.
This news article did not disclose which type of Ebola, Sudan or Zaire, was detected.
INOVIO today announced that Dr. Angela Huttner, Infectious Disease Consultant, Geneva University Hospitals presented data from a Phase 1b trial evaluating INO-4201, an Ebola booster vaccine candidate for rVSV-ZEBOV (Ervebo®).
"Preliminary data showed that INO-4201 was well tolerated and produced a strong immune response," stated Dr. Huttner in a press release on April 17, 2023.
"This suggests that a booster dose of IN0-4201 has the potential to extend protection against Ebola and could be an important tool in future Ebola Virus Disease prevention."
In February 2023, INOVIO announced positive initial results from the Phase 1b trial that evaluated INO-4201 as a booster in healthy adult participants who previously received a single injection of Ervebo.
These initial results showed that INO-4201 was well-tolerated and boosted humoral responses in 100% (36 of 36) of treated participants. In addition, data presented today included the assessment of binding antibodies showing that all 36 vaccine recipients responded to the boost.
The unedited press release is posted at this link.
Note: Merck's Ervebo® Vaccine is a live, recombinant, replication-competent vaccine approved by the U.S. FDA and by the European Medicines Agency.
Since 2019, approximately 300,000 persons have been vaccinated with the Ervebo vaccine in Africa.
Other Ebolavirus (Zaire and Sudan) vaccine and outbreak(s) news is posted at Vax-Before-Travel.
INOVIO today announced that an abstract had been accepted for presentation for INO-4201 as an Ebola booster for Merck's Ervebo® (rVSV-ZEBOV) vaccine at the 33rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID).
"We are pleased that lead investigator Dr. Angela Huttner will have the opportunity to share important new humoral and cellular response data at ECCMID from our recently completed Phase 1b trial of INO-4201 as an Ebola booster vaccine candidate for Ervebo," said Dr. Laurent Humeau, INOVIO's Chief Scientific Officer, in a press release on April 12, 2023.
INO-4201 is a DNA vaccine targeting Zaire Ebola virus (ZEBOV) glycoprotein, designed to prevent infection.
INO-4201 encodes for a synthetic consensus antigen encompassing ZEBOV genetic variability from various outbreak strains to broaden immune coverage for divergent ZEBOV variants.
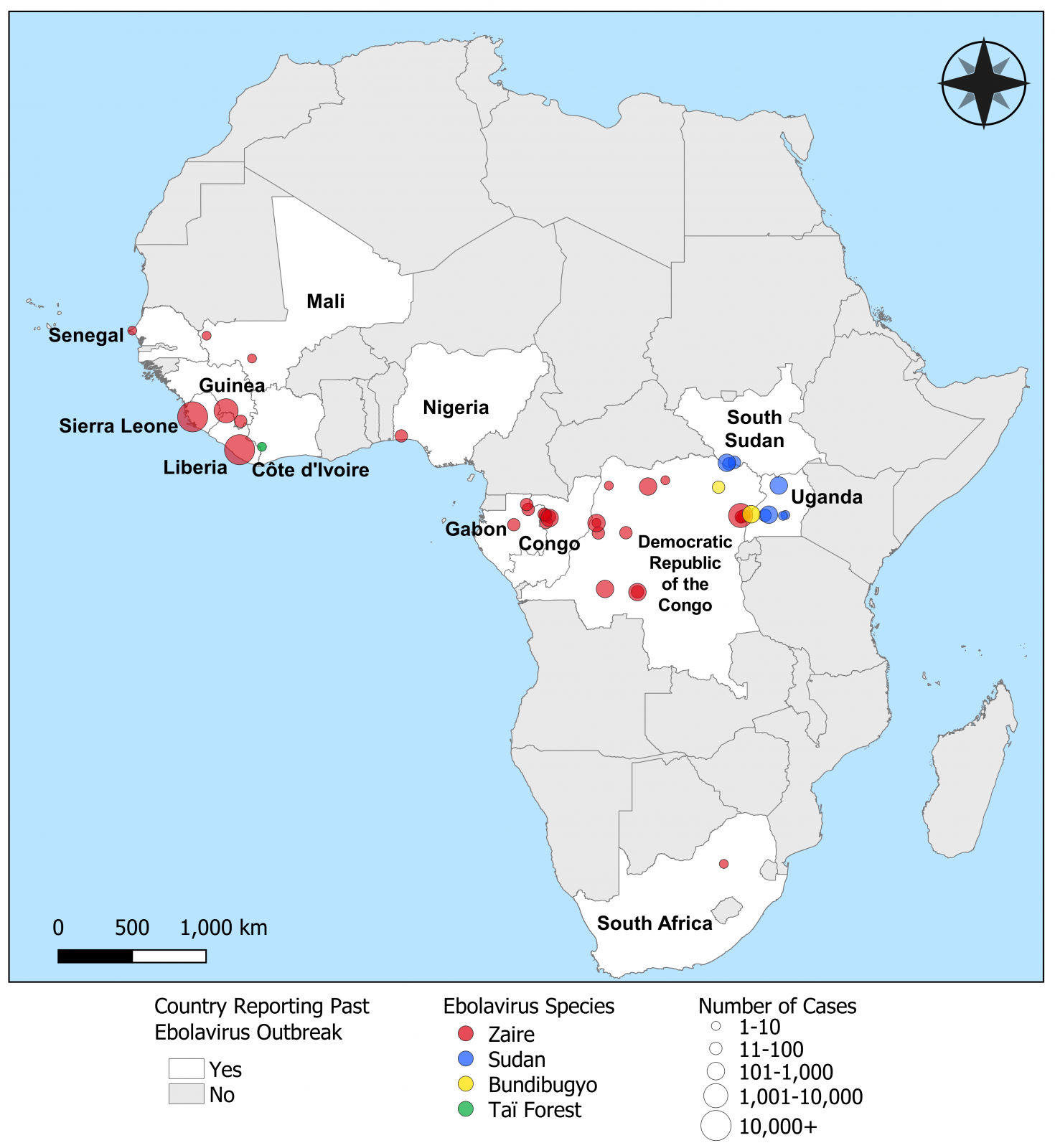
The Ebola virus family includes four virus species that cause periodic outbreaks of a highly contagious and lethal human infectious disease called Ebola Virus Disease (EVD).
New research suggests dormant Ebola virus in a previously infected survivor could re-emerge up to nearly five years later and again allow human-to-human transmission.
The Ebola virus is classified as a Category A Priority Pathogen by the U.S. Centers for Disease Control and Prevention.
Also, the World Health Organization lists EVD as a priority for research and development in emergency contexts and coordinates planning to prevent and respond to Ebola epidemics.
Ebola vaccines have been approved and deployed in Africa.

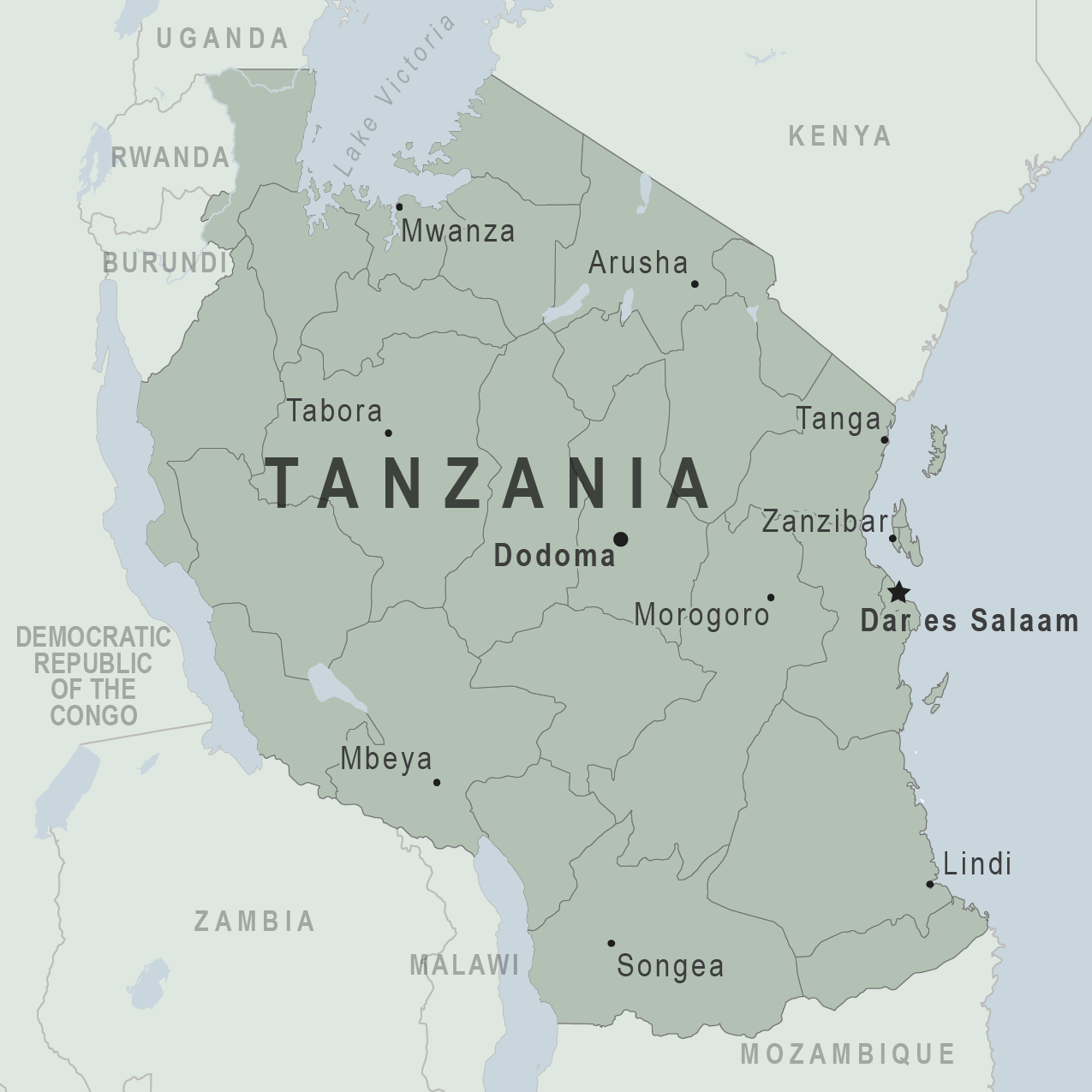
AfricaNews recently reported the United Republic of Tanzania requested medical experts investigate a mysterious "communicable" disease that has already killed five people in the country in March 2023.
Outbreaks are not new in the east African country of Tanzania.
In July 2022, a disease whose symptoms included nosebleeds, fever, headaches, and fatigue, was detected in the Lindi region. A total of 13 patients were detected then.
The World Health Organization later confirmed 20 cases of leptospirosis in two districts in the Lindi Region, including three deaths. The majority of these cases were men who were farmers as of August 8, 2022.
And in 2019, a disease with Ebola-like symptoms killed one woman who had visited Uganda, where an Ebola outbreak was detected.
Tanzania was formed as a sovereign state in 1964 through the combination of Tanganyika and Zanzibar, reports Britannica.
No U.S. CDC health notices are in effect for Tanzania, including Zanzibar, as of March 18, 2023.