Search API
In early October 2024, the Republic of Rwanda began vaccinating frontline health workers in a Phase 2 rapid response open-label clinical trial to combat the reaction to the ongoing Marburg virus disease (MVD) outbreak, which has already claimed 14 lives.
Sabin Vaccine Institute’s single-dose Marburg vaccine candidate was selected to be administered in accordance with the clinical protocol reviewed and approved by Rwandan ethics and regulatory authorities. However, this is not a U.S. FDA-approved vaccine.
As of October 12, 2024, Sabin announced it had delivered approximately 1,700 investigational vaccine doses to Rwanda.
“In an outbreak, every moment counts, and our seamless collaboration with the Rwandan government was key to accelerating the process. On our side, we moved quickly by leveraging our experience with other outbreaks and having vaccine doses and supporting documents ready, thanks to a strong partnership with ReiThera,” says Sabin's CEO Amy Finan in a press release.
Sabin has extensive expertise in advancing vaccines for filoviruses, with two programs currently in Phase 2 clinical trials—one for Marburg and the other for Sudan ebolavirus.
The U.S. government has obligated $235 million to Sabin to advance vaccine research and development against Sudan ebolavirus and MVD.
As of October 13, 2024, other MVD vaccine candidates are conducting clinical research.
Previously, the U.S. CDC announced that people should reconsider nonessential travel to the Republic of Rwanda and that those who arrive in the U.S. may be screened for the virus at certain airports.
According to Reuters, Gilead Sciences today announced it would donate about 5,000 vials of its antiviral drug remdesivir (Veklury®) for emergency use in response to the ongoing Marburg virus disease (MVD) outbreak in the Republic of Rwanda.
This U.S. FDA-approved antiviral drug is being evaluated since no approved MVD vaccines are available.
However, several Marburg vaccine candidates, utilizing various technologies, are actively conducting clinical trials.
The drugmaker clarified that Remdesivir, originally developed to treat Ebola, is not approved for treating Marburg disease anywhere, and its safety and efficacy against the virus is unknown.
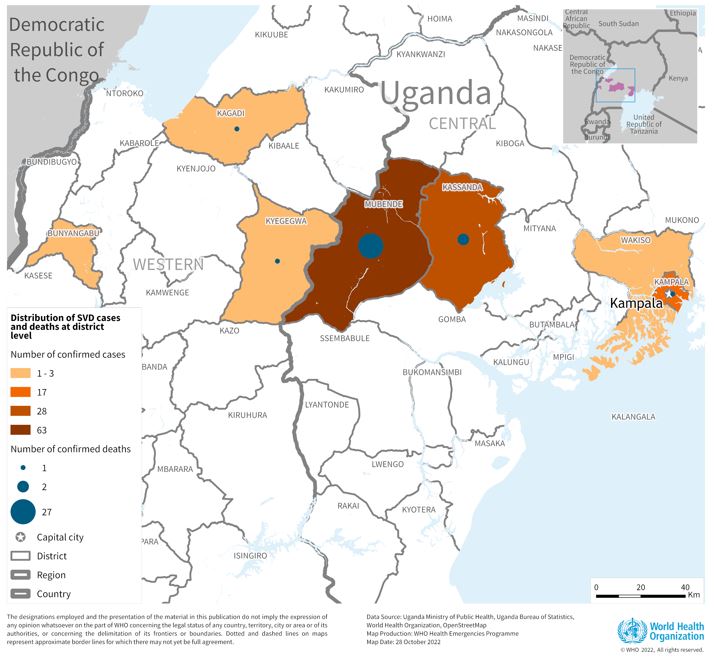
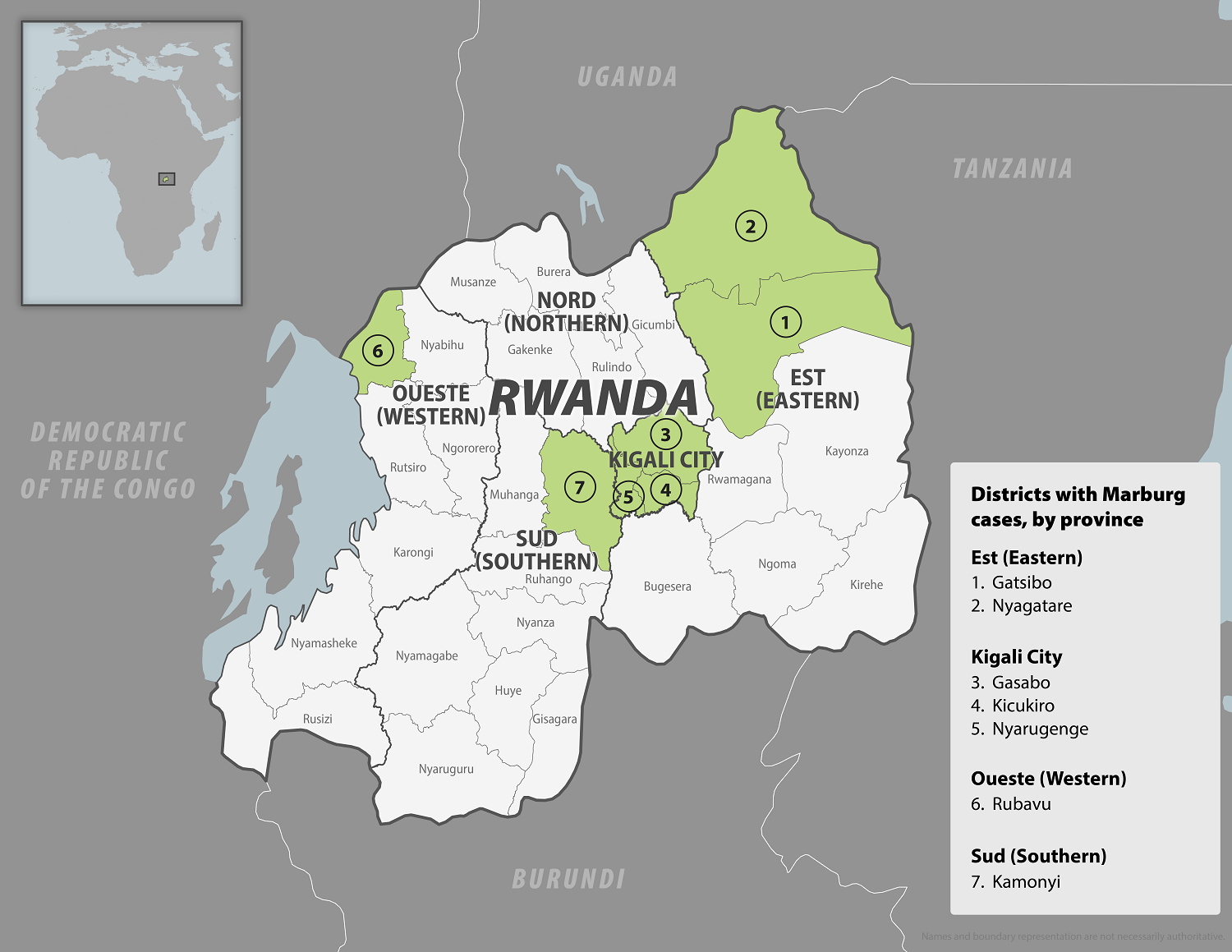
As of October 3, 2024, Rwanad's first MVD outbreak has produced eleven fatalities out of 36 confirmed cases.
The World Helath Organization recently assessed the risk of this MVD outbreak as very high at the national level, high at the regional level, and low at the global level. And the local government anticipates additional MVD cases to be reported this year.
The Republic of Rwanda's health ministry reported today that the sudden Marburg virus disease (MVD) outbreak had reached 29 cases and ten related fatalities.
On September 30, 2024, the World Health Organization confirmed MVD cases from seven of the 30 districts in Rwanda.
Over 70% of the confirmed cases are healthcare workers from two health facilities in Kigali, a city with about 1.5 million residents.
On September 27, 2024, the Rwanda Ministry of Health reported its first Marburg disease case.
Additionally, the U.S. CDC published a Travel Health Advisory to alert international travelers of this expanding health risk. Marburg virus is a Filovirus that, along with Ebola, can cause severe viral hemorrhagic fever infections. MVD was initially reported in 1967 during an outbreak in Marburg a der Lahn and Frankfurt am Main, West Germany.
As of October 1, 2024, no Marburg vaccine candidate has been approved to prevent infections in people.
According to local media Taarifa, the Rwanda Ministry of Health reported its first Marburg virus disease outbreak today.
As of September 27, 2024, Rwanda's communique stated a 'few' Marburg cases are being investigated. The Ministry added that the situation is closely monitored and that further updates will be provided regularly.
Like Ebolavirus, Marburg virus disease is highly virulent and causes hemorrhagic fever, with a fatality ratio exceeding 80%.
Marburg cases were first recognized in 1967 in West Germany and Serbia.
As of September 2024, Angola, DR Congo, Equatorial Guinea, Cameroon, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, Tanzania, Uganda, and Rwanda have previously confirmed Marburg cases.
The World Health Organization published the Marburg virus vaccine development landscape on February 13, 2023. While various vaccine candidates conduct clinical research, no approved vaccines prevent Marburg virus infections.
Unlike Marburg, approved vaccines that prevent and treat Zaire Ebola disease are available in 2024.
Albert Einstein College of Medicine recently announced it received a five-year, $14 million per year grant from the National Institute of Allergy and Infectious Diseases (NIAID) to participate in a broad national effort to develop "plug-and-play" vaccines and antibody-based therapies against a wide range of emerging viruses.
The Einstein-led consortium, called PROVIDENT (Prepositioning Optimized Strategies for Vaccines and Immunotherapeutics Against Diverse Emerging Infectious Threats), will link 13 teams in academia, government, and industry that will conduct four projects designed to:
Discover and analyze virus-host interactions and the molecular mechanisms involved in viral disease,
Design proteins to elicit antiviral immune responses and then evaluate and optimize those responses,
Create “road maps” for quickly developing RNA vaccines against microbes with pandemic potential, and
Map the antibody responses observed in people infected with viruses and use this knowledge to design vaccines and therapeutics.
PROVIDENT builds on NIAID’s 2021 Pandemic Preparedness Plan, which focuses on “priority pathogens” and “prototype pathogens.” Priority pathogens include viruses known to cause significant human illness or death, such as dengue and Ebola.
“Recent outbreaks of mpox, Nipah virus, and Eastern equine encephalitis, among other viral infections, underscore the need for an even broader preparedness program,” said Eva Mittler, Ph.D., research assistant professor at Einstein and leader of one of the PROVIDENT components, in a press release on September 13, 2024.
“We don't know what virus will cause the next pandemic.”
The $70 million grant is part of NIAID’s new Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness (ReVAMPP) Network.
The ReVAMPP network focuses on viruses from the Flaviviridae family, which features viruses that cause dengue and yellow fever; the Paramyxoviridae family, which contains viruses that cause measles, mumps, and Nipah-induced encephalitis; the Picornaviridae family, whose members cause poliomyelitis, foot-and-mouth disease, and myocarditis; the Togaviridae family, which contains viruses that induce Chikungunya virus-induced arthralgia or encephalitis and Venezuelan equine encephalitis; as well as viruses from 5 different families within the Bunyavirales order, including Sin Nombre virus from the Hantaviridae family and the viruses that cause Rift Valley Fever (Phenuiviridae), Crimean Congo Hemorrhagic Fever (Nairoviridae), Oropouche Fever (Peribunyaviridae), and Lassa Fever (Arenaviridae).
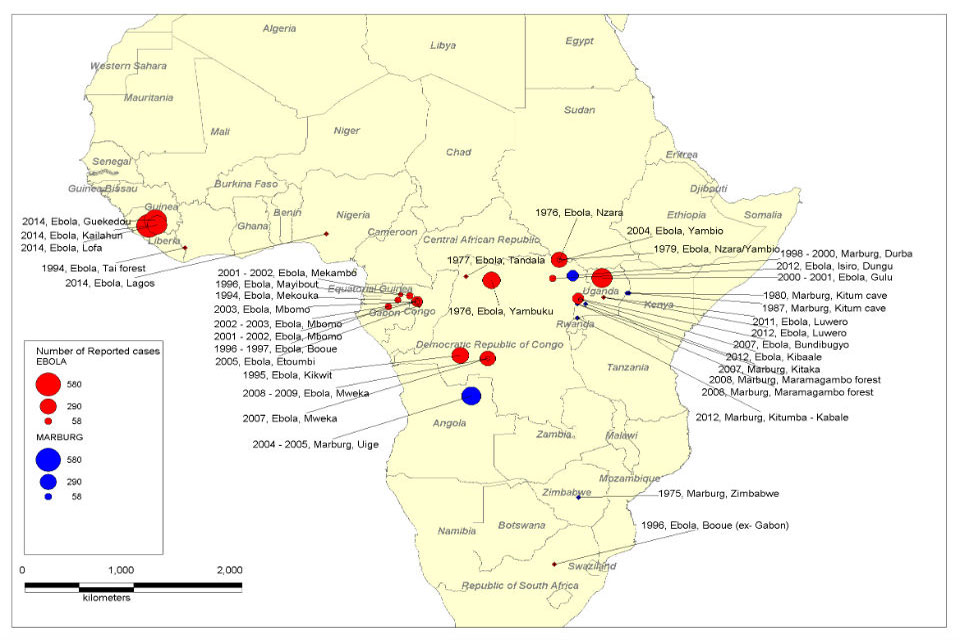
While there is an approved Ebola virus disease (EVD) vaccine, the U.S. government continues to invest in human monoclonal antibody (mAb) therapy during Zaire ebolavirus outbreaks in Africa.
The initial Zaire Ebolavirus disease (EVD) case was confirmed in 1976, Since then, more than 30 EVD outbreaks have been reported.
Emergent BioSolutions Inc. announced today that it was awarded a contract modification executing an option period by the Biomedical Advanced Research and Development Authority (BARDA), valued at $41.9 million, for drug substance engineering and scale-up process validation, long-term stability, and commercial readiness in support of its ongoing scale-up program for Ebanga™, a licensed glycoprotein (EBOV GP)-directed mAb treatment for EVD.
“Emergent is proud to continue to advance the Ebanga™ (ansuvimab-zykl) development and scale up to its next phase,” said Paul Williams, senior vice president of products business, Emergent, in a press release on September 12, 2024.
This mAb binds to a portion of the Ebola virus's surface called the glycoprotein, which prevents the virus from entering a person's cells. Ebanga's efficacy has not been established for other species of the Ebolavirus and Marburgvirus.
Under the terms of the contract, Emergent will complete activities to advance the development of Ebanga™ treatment through post-licensure commitments, including the transfer of technology as part of manufacturing scale-up, submission of a supplemental Biologics License Application to the U.S. Food and Drug Administration (FDA), and completion of stability studies.
The existing 10-year contract consists of a base period of performance with two option periods for advanced development valued at approximately $121 million and option periods for procurement of Ebanga™ treatment over five years valued at up to $583 million. Execution of this option period is in line with Emergent’s planned program performance and critical path for developing the Ebanga™ treatment.
BARDA is part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
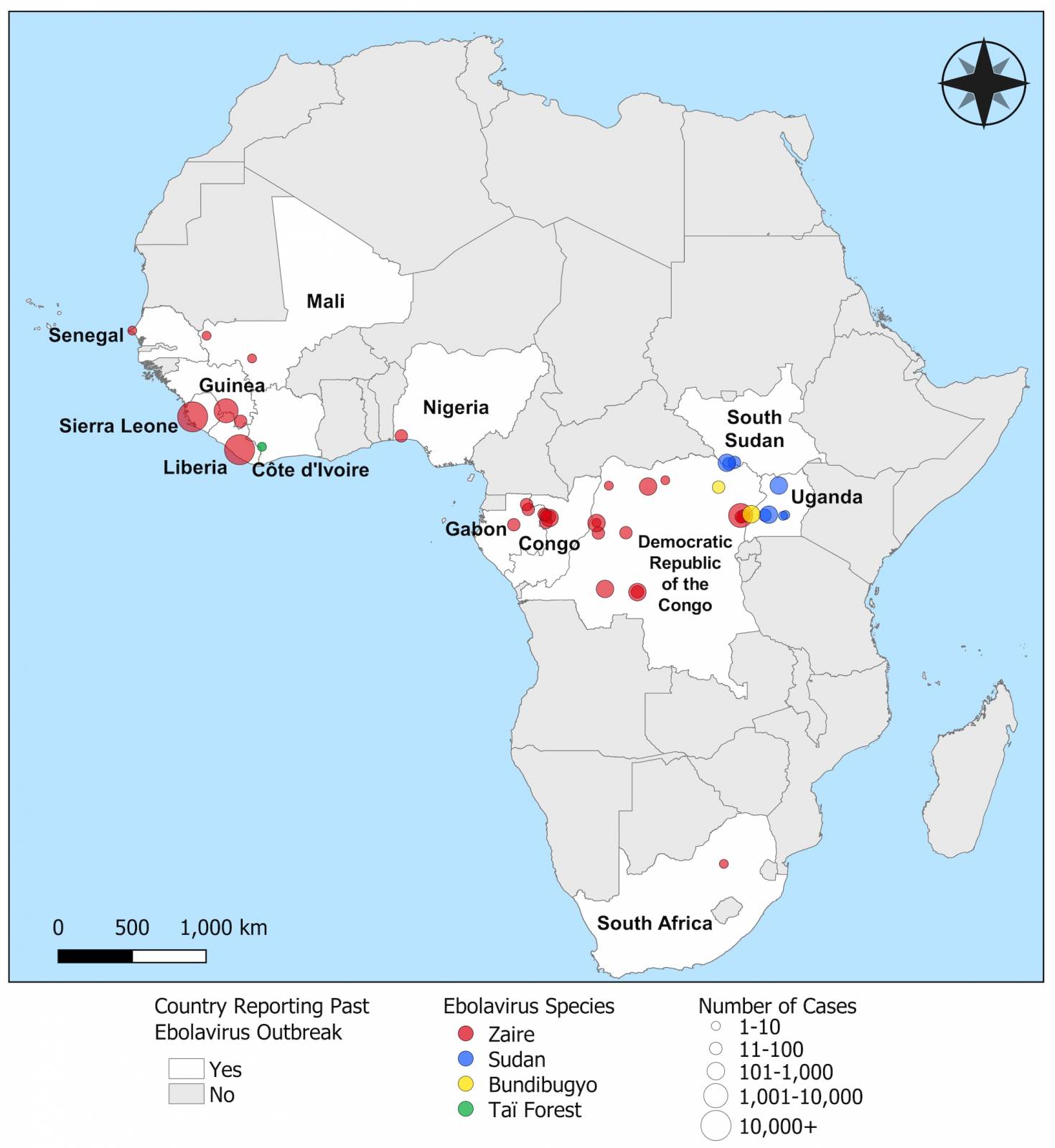
According to real-world evidence published in The Lancet Infectious Diseases today, this analysis is the first to provide estimates of Merck's Ervebo® (rVSV-ZEBOV) vaccine against Zaire Ebolavirus disease amid the widespread use of the vaccine during a large outbreak.
Announced on August 20, 2024, these findings confirm that Ervebo is highly protective against 84% (95% credible interval, 70% to 92%) of Ebolavirus disease and supports its use during outbreaks, even in challenging contexts such as in the eastern Democratic Republic of the Congo (DRC).
This finding is essential since Ebolaviruses are endemic in the DRC.
In a related Editorial, the authors wrote the 2018–20 Ebola virus disease epidemic in the DRC resulted in 3,470 reported cases and remains the second-largest Ebolavirus outbreak in recorded history worldwide. The initial Ebola outbreak was in 1976.
In November 2019, the World Health Organization prequalified the Ervebo vaccine. The U.S. Food and Drug Administration approved it on December 19, 2019.
Médecins Sans Frontières (Doctors Without Borders) funded this study.
The Sabin Vaccine Institute recently announced the launch of a Phase 2 clinical trial for its vaccine against Sudan ebolavirus at Makerere University Walter Reed Project (MUWRP) in Uganda.
This is a vital development as there are currently no approved vaccines for this strain of ebolavirus.
Based on the cAd3 platform, Sabin’s single-dose investigational Sudan ebolavirus vaccine was found to be promising in Phase 1 clinical and non-clinical studies. Results showed it to be safe while eliciting rapid and robust immune responses that lasted up to 12 months.
“We are delighted to advance a vaccine candidate that can thwart a deadly and devastating disease, especially one that caused a fairly recent outbreak and for which no approved treatments exist,” commented Amy Finan, Sabin’s Chief Executive Officer, in a press release on July 15, 2024.
“Sabin’s vaccine candidate is backed by strong safety and immunogenicity data, and we hope this trial will yield further evidence to move the vaccine closer to licensure.”
This is Sabin’s second Phase 2 clinical trial partnership with MUWRP, based in Uganda’s capital, Kampala. A Phase 2 trial for a Marburg vaccine is already underway, having recently completed enrollment. Initial results from the Marburg trial are expected later this year.
The most recent outbreak of Sudan ebolavirus occurred in Uganda in the fall of 2022. That outbreak ultimately resulted in 55 deaths.
Sabin’s vaccine candidate was the first to arrive in Uganda during that outbreak after the WHO included it as one of three vaccines for possible use in an outbreak trial. The outbreak ended before the vaccine was deployed.
In August 2019, Sabin announced agreements with GSK to advance the development of vaccines against the Zaire and Sudan ebolavirus and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, acquired by GSK in 2013.
As of July 20, 2024, the U.S. FDA has approved Zaire Ebolavirus vaccines, which have been offered in Africa since 2019.