Search API
A meeting of the U.S. FDA's Peripheral and Central Nervous System Drugs Advisory Committee is scheduled for June 9, 2023.
This FDA Committee's digital presentation will discuss the supplemental biologics license application for LEQEMBI™ (lecanemab) solution for intravenous infusion, submitted by Eisai, Inc., for treating early Alzheimer's disease (AD).
The Committee will discuss the confirmatory study, BAN2401-G000-301, conducted to fulfill post-marketing requirement 4384-1, detailed on January 6, 2023, FDA approval letter.
Confirmatory studies verify and describe a product's clinical benefit after receiving an FDA accelerated approval. Accordingly, its application was granted Priority Review, with a Prescription Drug User Fee Act action date of July 6, 2023.
LEQEMBI is a humanized immunoglobulin gamma 1 monoclonal antibody, not a preventive vaccine.
As of April 15, 2023, the FDA has not approved any Alzheimer's vaccine candidate.
FDA advisory committees provide independent expert advice on topics or specific products to help the agency make sound decisions based on the available science. Advisory committees make non-binding recommendations.
The FDA generally follows these recommendations but is not legally bound to do so.

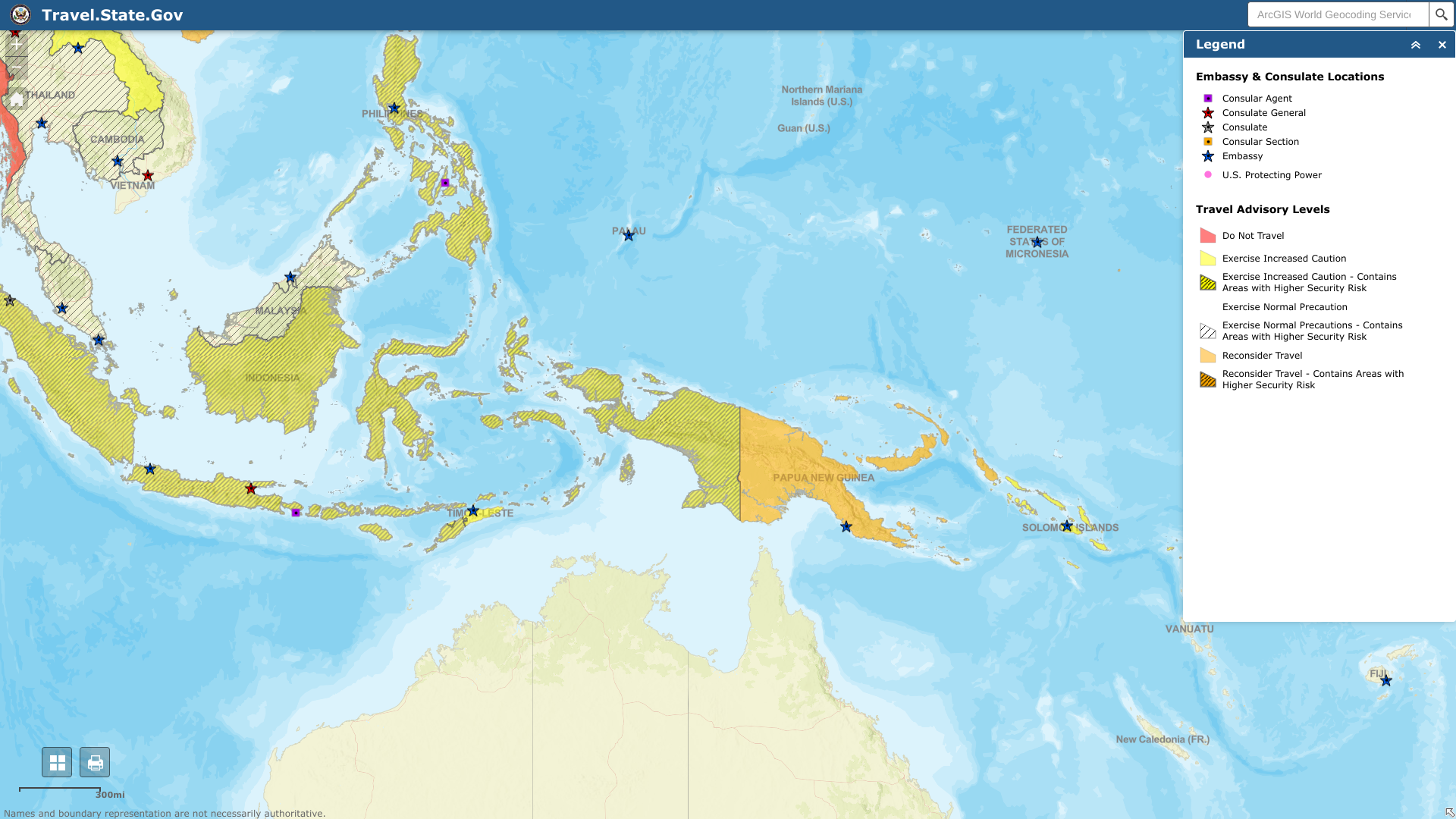
The U.S. Department of State reissued its Level 3: Reconsider Travel for the Independent State of Papua New Guinea.
On April 11, 2023, the State Department announced travelers should reconsider visiting Papua New Guinea due to crime, civil unrest, and piracy.
U.S. government employees must obtain authorization before traveling to areas of concern, including the southern part of Bougainville and the provinces of Southern Highlands, Western Highlands (excluding Mt. Hagen), Eastern Highlands (excluding Goroka), Hela, Enga, Jiwaka, and other areas of Papua New Guinea where one is unable to fly directly.
Additionally, the Travel Advisory says 'do not travel' to:
- Southern Bougainville, particularly areas near the Panguna mine.
- The Highlands region, other than the towns of Mt. Hagen and Goroka.
And there have been reports of criminals attacking resorts popular with foreign tourists to steal goods and money. And police presence is limited outside of the capital, Port Moresby.
Furthermore, piracy is active in the waters surrounding Papua New Guinea, located in the eastern area of New Guinea, the world's second-largest island. The western half of the island is part of Indonesia.
Travelers by boat should reconsider travel to the Bismarck and Solomon Seas along Papua New Guinea's north and eastern coasts. In 2021 and 2022, the Embassy was aware of at least three occasions in which sailboats operated by or carrying U.S. citizens were boarded by criminals and, in one incident, severely injured the captain.
From a health perspective, the U.S. CDC suggests various travel vaccines before visiting Papua New Guinea.
The Weekly Influenza Surveillance Report #14, published today by the U.S. Centers for Disease Control and Prevention (CDC), indicates good news for the USA.
As of April 14, 2023, the CDC says seasonal influenza activity remains low nationally, with eight of 10 regions below their respective baselines.
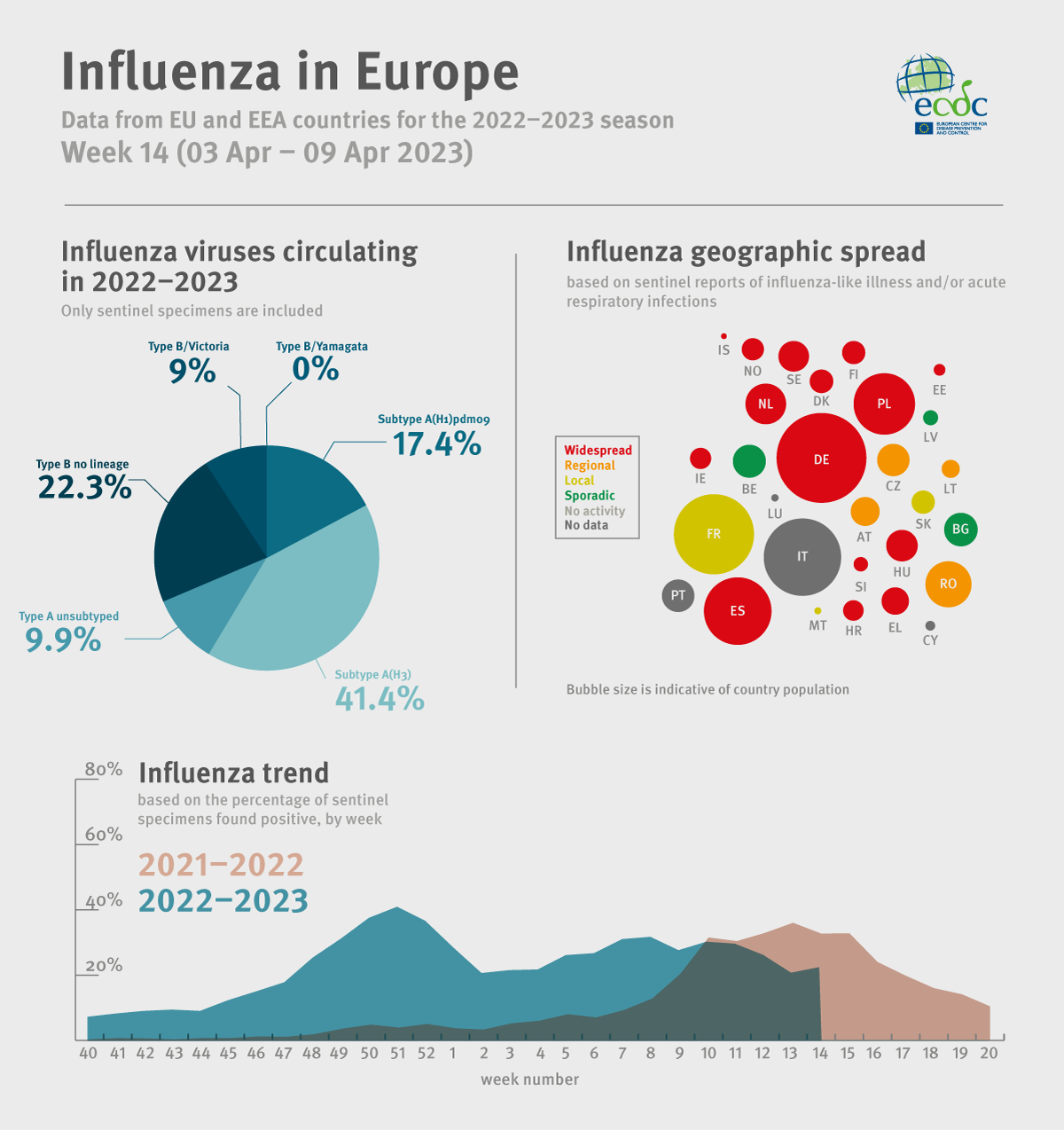
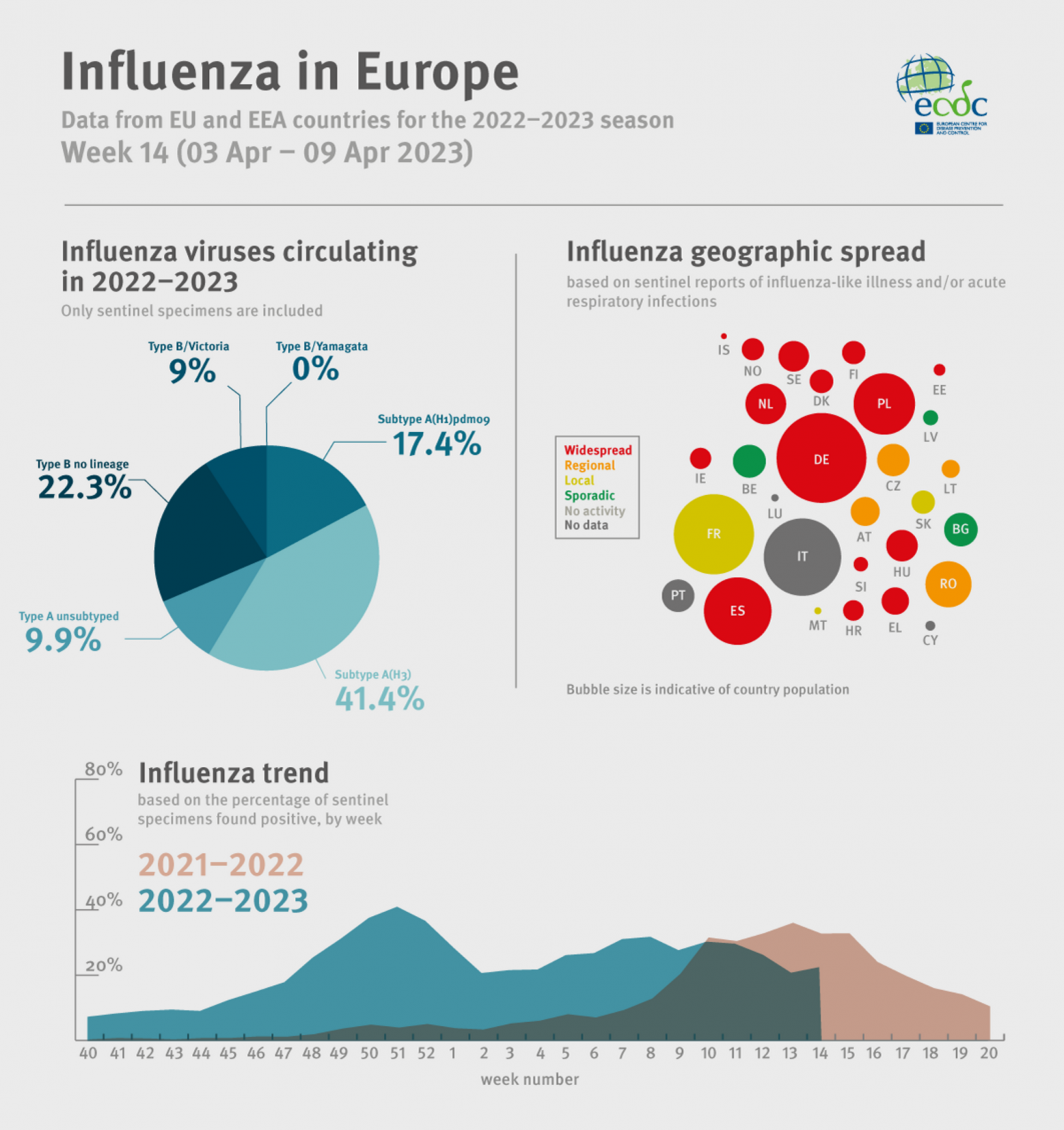
However, the European CDC reported on April 14, 2023, there was a 15% influenza positivity for week 14/2023.
Of 41 countries and areas in Europe reporting on the spread of influenza viruses, 16 reported widespread activity across the Region.
The CDC continues to suggest anyone concerned about respiratory viruses should speak with a healthcare provider regarding their flu shot option.
U.S. HHS Secretary Becerra today announced that in the coming weeks, he would issue an amendment to the declaration under the Public Readiness and Emergency Preparedness (PREP) Act for medical countermeasures against COVID-19.
In light of the significant impact of the PREP policy on the healthcare landscape and to provide further clarity, HHS offered additional information about key elements of its plan's flexibilities and protections on April 14, 2023, that will remain in place moving forward.
For example, extending protection coverage for COVID-19 vaccines, seasonal influenza vaccines, and COVID-19 tests will be enhanced.
And PREP Act immunity from liability will be extended through December 2024 to pharmacists, pharmacy interns, and pharmacy technicians to administer COVID-19 and seasonal influenza vaccines and COVID-19 tests, regardless of any USG agreement or emergency declaration.
In the month remaining before the end of the COVID-19 Public Health Emergency, HHS stated it would continue to work closely with its partners, including Governors, state, local, Tribal, and territorial agencies, industry, and advocates to ensure an orderly transition.
Additional, unedited information is posted at this HHS link.

The European Centre for Disease Prevention and Control (ECDC) recently published an influenza update for week #14, indicating flu positivity was about 15%.
Announced on April 14, 2023, the ECDC confirmed of the 41 countries and areas reporting data on influenza viruses:
- 3 reported no activity (Georgia, Kazakhstan, and Kyrgyzstan),
- 9 reported sporadic spread (eastern, northern, and southern Region),
- 5 reported local spread (France, Malta, Serbia, Slovakia and Kosovo,
- 8 reported regional spread (Albania, Austria, Bosnia and Herzegovina, Czechia, Lithuania, Republic of Moldova, Romania and Ukraine, and,
- 16 reported widespread activity (across the Region).
The ECDC added seasonal influenza is a vaccine-preventable disease that annually infects over 10% of Europe's population.
When planning a European visit, the U.S. Centers for Disease Control and Prevention suggests discussing flu shot options with a healthcare provider.
SAB Biotherapeutics today announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track designation for SAB-176, an investigational therapeutic for Type A and Type B influenza illness in high-risk patients, including those who have anti-viral resistant strains.
SAB-176 offers the potential for additional treatment for influenza, particularly in higher-risk patients.
SAB also received FDA guidance and regulatory alignment on advancing SAB-176 into the next development phase by initiating a Phase 2b dose-range finding efficacy and safety trial in high-risk patients for developing severe disease.
SAB-176 is a novel, highly potent immunotherapy grounded in the fundamentals of the natural immune response to neutralize Type A and Type B influenza viruses, which mutate rapidly.
SAB-176 has undergone multiple clinical and pre-clinical studies, including a Phase 1 trial in healthy volunteers and a Phase 2a challenge study completed last year.
In the Phase 2a study, SAB-176 showed broad cross-protection that included influenza strains not explicitly targeted in manufacturing the therapeutic.
"We are pleased to receive the FDA Fast Track designation for SAB-176. Influenza continues to be one of the biggest public health challenges the world faces continuingly, with an excessively high number of hospitalizations and deaths each year," said Eddie Sullivan, Ph.D., co-founder, President & CEO of SAB Biotherapeutics, in a press release on April 13, 2023.
"We are excited about the potential role SAB-176 can play in tackling a highly mutagenic pathogen like influenza."
SAB-176 is also being studied in emerging and mutating pandemic strains by targeting multiple epitopes of the virus rather than a single epitope.
While Tamiflu® is an effective therapy for treating influenza if used within two days of symptom onset, some patients still develop severe disease and resistant strains of influenza to anti-viral drugs.
Throughout the 2022-2023 flu season in the U.S., over 171 million influenza vaccines were distributed, which remain available at health clinics and pharmacies.

The Florida Health Department reported as of week #13, there had been 59 travel-associated dengue cases. And, as of April 8, 2023, there are now 2 locally acquired dengue cases confirmed in 2023.
In 2022, Florida reported 903 travel-associated and 68 locally-acquired dengue cases.
In the Region of the Americas, 46 countries and territories reported dengue cases in 2022. For example, dengue was reported in 28 of 32 Mexico states last year.
These countries confirmed about 2.8 million dengue cases, representing a two-fold increase compared to 2021.
Dengue is a vaccine-preventable disease, and as of April 13, 2023, two vaccines are authorized in various countries.

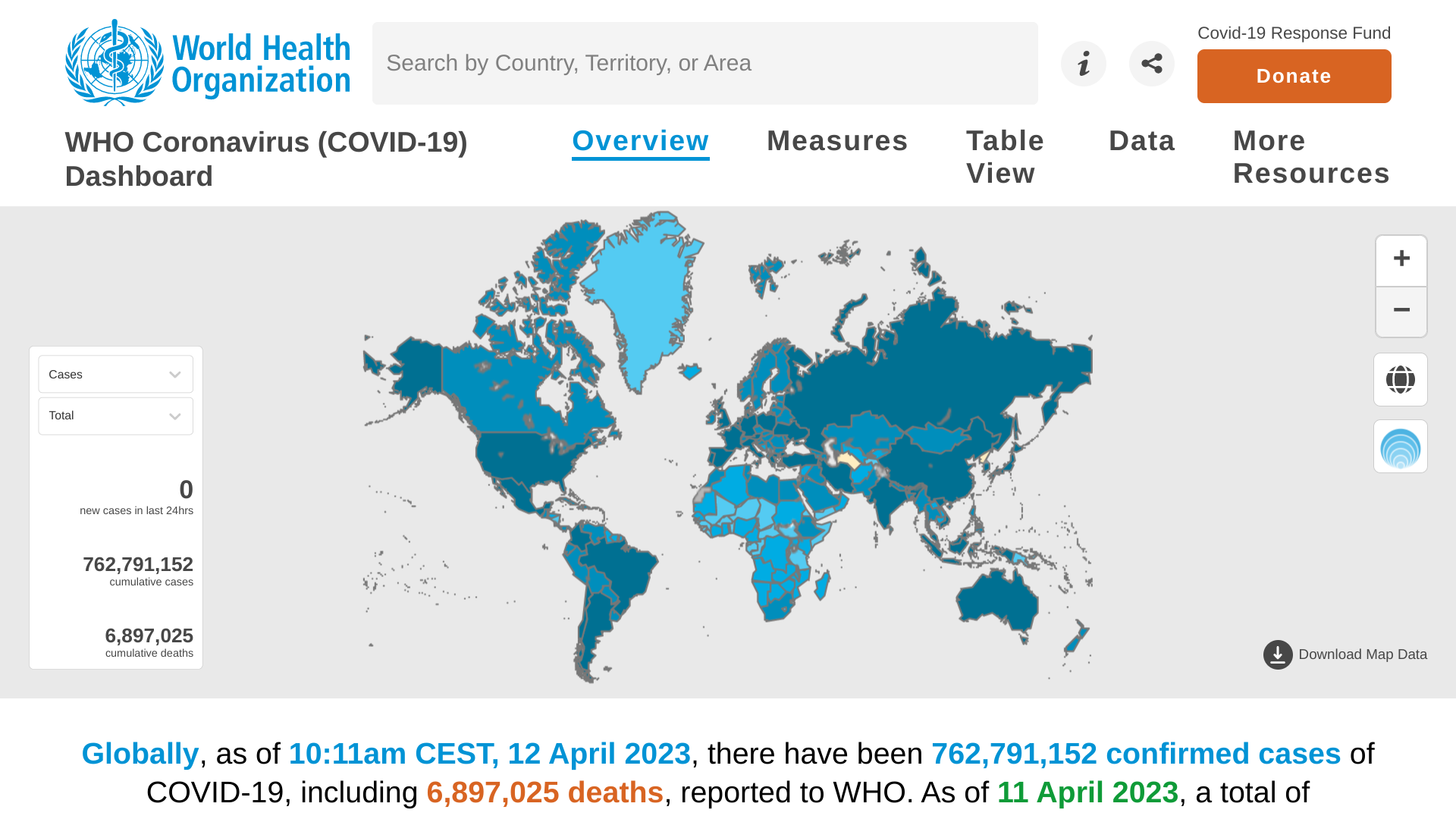
The World Health Organization (WHO) today published its weekly epidemiological update focused on the COVID-19 pandemic.
On April 13, 2023, the WHO's Edition #138 highlighted very positive trends.
Over the last 28 days (March 13 to April 9, 2023), COVID-19 cases decreased by 28%.
And related fatalities declined by 30% compared to the previous period.
However, contrary to the global trend, increases in reported COVID-19 cases and deaths were seen in the South-East Asia and Eastern Mediterranean regions and several individual countries.
At the regional level, the number of newly reported 28-day cases decreased across four of the six WHO regions: The African Region (-45%), the Western Pacific Region (-39%), the Region of the Americas (-33%), and the European Region (-22%).
While case numbers increased in two WHO regions: the South-East Asia Region (+481%) and the Eastern Mediterranean Region (+144%).
The highest numbers of new 28-day cases were reported at the country level from the U.S., the Russian Federation, the Republic of Korea, Brazil, and France.
The WHO suggests international travelers remain fully protected against COVID-19 by speaking with a healthcare provider to determine if a Spring or Summer COVId-19 booster is recommended, as well as various travel vaccines.