Search API
Valneva SE today reaffirmed that the Phase 3 clinical trial of its Lyme disease vaccine candidate, VLA15, remains on track.
The company's press release on October 6, 2025, states that Participants in the VALOR clinical trial will be monitored for the occurrence of Lyme disease cases until the end of 2025. Valneva expects the VALOR trial outcomes to be announced in the first half of 2026, followed by planned regulatory submissions.
Valneva's development partner, Pfizer Inc., continues to aim to submit a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) and a Marketing Authorization Application to the European Medicines Agency in 2026, pending the receipt of positive Phase 3 data.
Pending approval, Valneva expects Pfizer to launch the vaccine in the second half of 2027.
The FDA granted the VLA15 vaccine development program Fast Track designation in July 2017, and it remains the leading candidate in development.
The VLA15 vaccine protects humans by raising antibodies that prevent Borrelia from migrating from ticks after a bite. VLA15 is designed to cover about 97% of Borrelia in North America and Europe. VLA15 is being tested as an alum-adjuvanted formulation and administered intramuscularly.
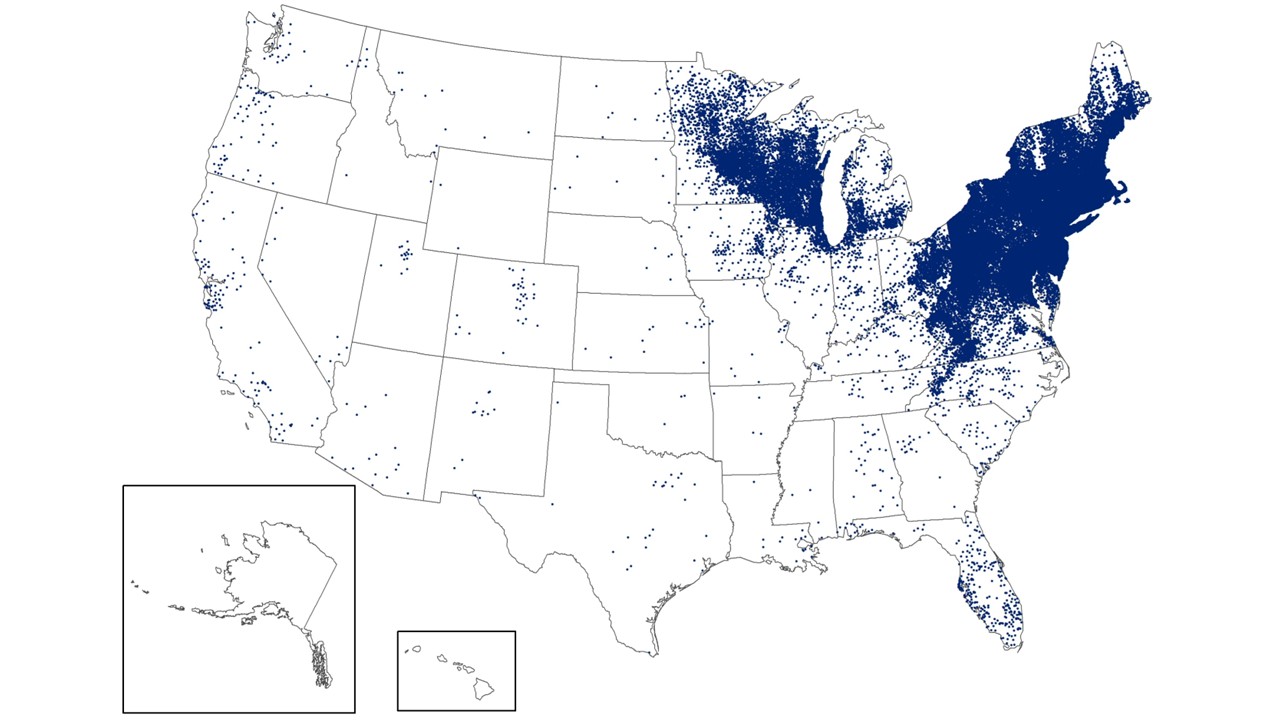
While Lyme disease has been found in the northeastern USA for decades, ticks in the upper Midwest are now spreading this severe disease.
According to the World Health Organization (WHO), India is the tuberculosis (TB) capital of the world, reporting about 2.5 million new cases annually. TB is the country's most fatal infectious disease, with an estimated 500,000 related fatalities every year.
The WHO says eliminating TB depends on early, accurate, and universal detection to reduce community transmission of this airborne disease.
To help reduce this significant health issue, the Indian Council of Medical Research has recently validated innovative tools from Huwel Lifesciences: the Quantiplus MTB FAST detection kit and the UniAMP MTB Nucleic Acid Test Card.
A recent study concluded that the Quantiplus assay demonstrated sensitivity and specificity of 86% and 96%, respectively, for the detection of pulmonary Mycobacterium tuberculosis (M. tuberculosis) in sputum samples, compared to liquid culture, and showed significant improvement with the Xpert MTB/RIF assay.
The diagnostic performance of the Quantiplus® assay is comparable to that of the Truenat MTB assay reported earlier. The limitation of the assay is that it requires a clean environment to avoid cross-contamination.
These new technologies promise to transform TB detection, making it faster, more affordable, and broadly accessible across India.
The 100-year-old Bacillus Calmette-Guérin (BCG) vaccine is the primary TB vaccine used in India. BCG provides partial protection against TB infection, especially in high-risk populations. It is administered to infants as part of the National Immunization Program.
India is actively involved in the development of new TB vaccines, such as MTBVAC and VPM1002. These vaccine candidates aim to provide broader and more durable protection against TB.
In the United States, access to the FDA-approved BCG vaccine is limited even as TB cases continue to increase.

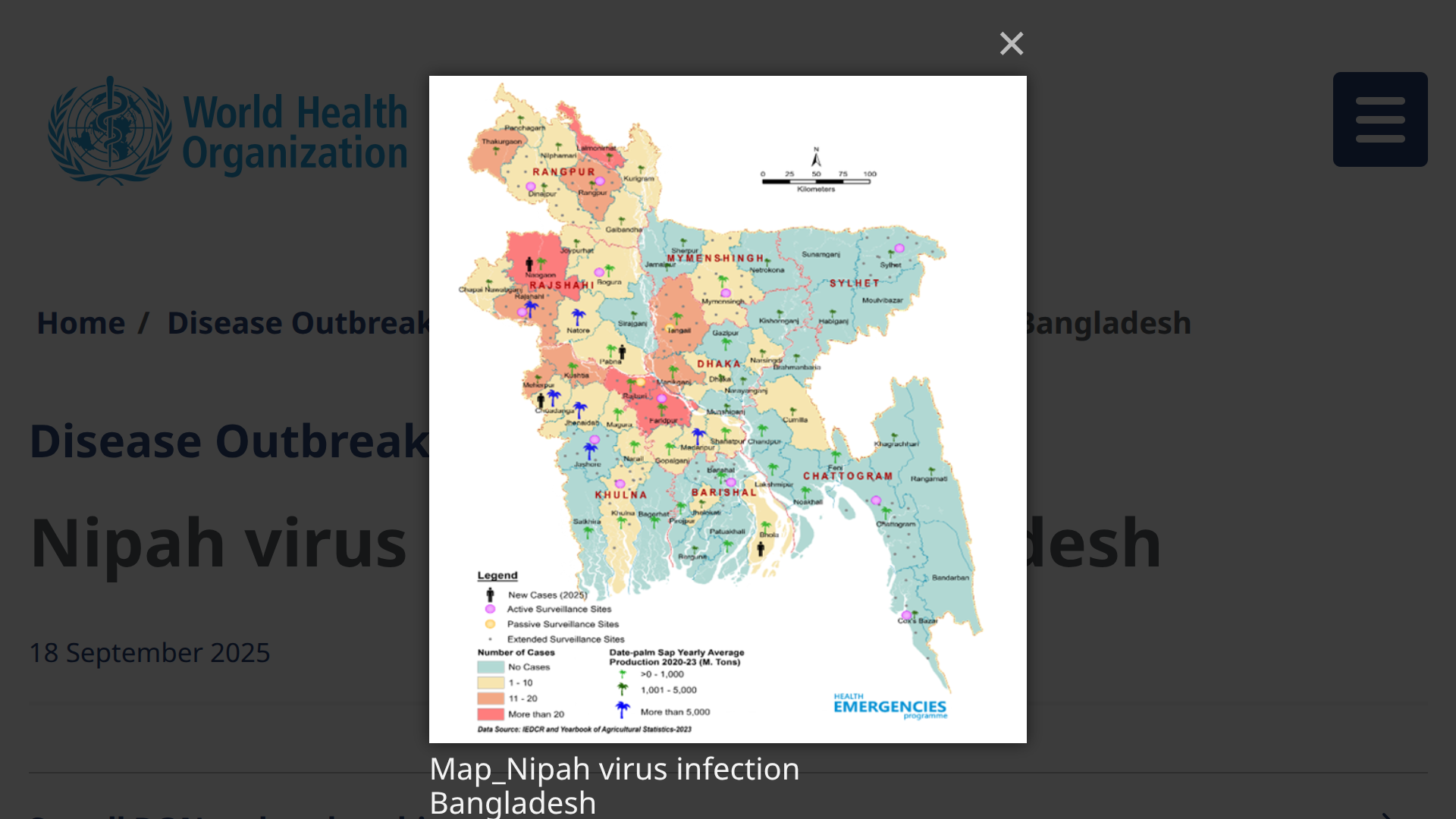
The International Health Regulations National Focal Point (IHR NFP) for Bangladesh recently notified the World Health Organization (WHO) of four confirmed fatal cases of Nipah virus (NiV) infection.
As of September 18, 2025, Bangladesh has documented 347 NiV cases through its Nipah surveillance system, which was established to detect and respond to outbreaks promptly, with a case fatality rate of 71.7%.
The WHO reported that between January and August 29, 2025, three geographical divisions in Bangladesh —namely, Barisal, Dhaka, and Rajshahi —reported these NiV patients.
Since the first recognized outbreak in Bangladesh in 2001, human NiV infections have been detected almost every year, says the WHO.
The Ministry of Health and Family Welfare in Bangladesh has implemented several public health measures with support from the WHO. The WHO assesses the overall public health risk posed by NiV at the national and regional levels to be moderate; the risk of international disease spread is considered low.
Human NiV infection is an epidemic-prone disease that can cause severe disease in humans and animals, with a high mortality rate, and outbreaks primarily occur in South and South-East Asia.
Recently, in India, NiV-related fatalities were reported.
As of August 6, 2025, Kerala State health officials have reported four cases to the WHO since mid-May, two of which have been fatal.
Since 2018, Kerala has experienced nine outbreaks of the Nipah virus, which is part of a pattern of recurring spillovers.
The WHO states there are currently no specific drugs or vaccines for NiV infection; intensive supportive care is recommended to treat severe respiratory and neurologic complications.
In 2023, the Coalition for Epidemic Preparedness Innovations invested $$100 million in four Nipah vaccine candidates.
Recently, the U.S. government announced a project to support the development of a Nipah monoclonal antibody, which is currently undergoing Phase 1 clinical trial testing in India and Bangladesh.
West African Ministers of Health today pledged their joint commitment to advance the development of, and readiness for, much-needed vaccines against Lassa fever.
First identified in 1969 in Nigeria, Lassa fever has a devastating impact on local populations.
Currently, there are no licensed vaccines to protect against Lassa fever.
As of September 8, 2025, these Ministers of Health are supported by the Coalition for Epidemic Preparedness Innovations (CEPI) and the International AIDS Vaccine Initiative (IAVI).
IAVI has developed the most advanced Lassa fever vaccine candidate, funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
IAVI's promising vaccine candidate is currently being evaluated in a Phase IIa clinical trial in Ghana, Liberia, and Nigeria, the most advanced study of a Lassa fever vaccine to date.
According to the WHO, Lassa fever, an acute viral illness, is endemic in Benin, Ghana, Guinea, Liberia, Mali, Nigeria, and Sierra Leone, but is likely also present in other West African countries.
The Lassa virus is primarily transmitted to humans via contact with food or household items contaminated with rodent urine or faeces. Person-to-person transmission can also occur, particularly in healthcare settings that lack adequate infection prevention and control measures.
The overall case fatality rate is 1%, but the observed case fatality rate among patients hospitalized with severe Lassa fever is 15% or higher.
As of week #33 in 2025, there were no Lassa fever cases in the USA>
In response to the United States' recent naval operation off the coast of the Bolivarian Republic of Venezuela, vacationers to the southern Caribbean Islands may make alternative plans this fall season.
These vacation destinations include Trinidad and Tobago, Grenada, Barbados, and the islands of Aruba, Curaçao, and Bonaire.
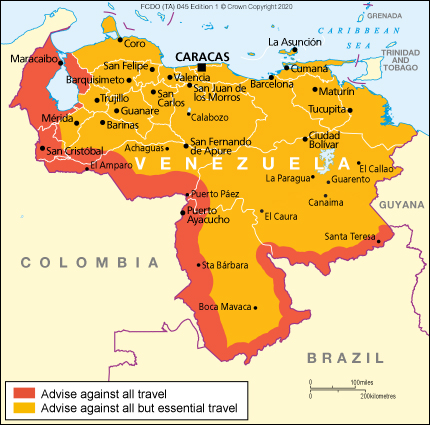
As of September 7, 2025, both the US Department of State and the UK government have updated their high-level travel advisories for Venezuela.
The US State Department writes, Do not travel to or remain in Venezuela due to the high risk of wrongful detention and civil unrest. All US citizens and Lawful Permanent Residents in Venezuela are strongly advised to depart immediately.
Additionally, no US embassy or consulate is operating in Venezuela, and the US government cannot provide routine or emergency consular services to US citizens in Venezuela.
Previously, the UK Foreign, Commonwealth & Development Office advised against travel within 80km of the Venezuela-Colombia border.
From a health perspective, if you plan to visit Venezuela in 2025, the US Centers for Disease Control and Prevention (CDC) recommends various routine and travel vaccinations before traveling to this South American country.
And since March 2025, the CDC has reported cases of Oropouche virus disease in Venezuela.