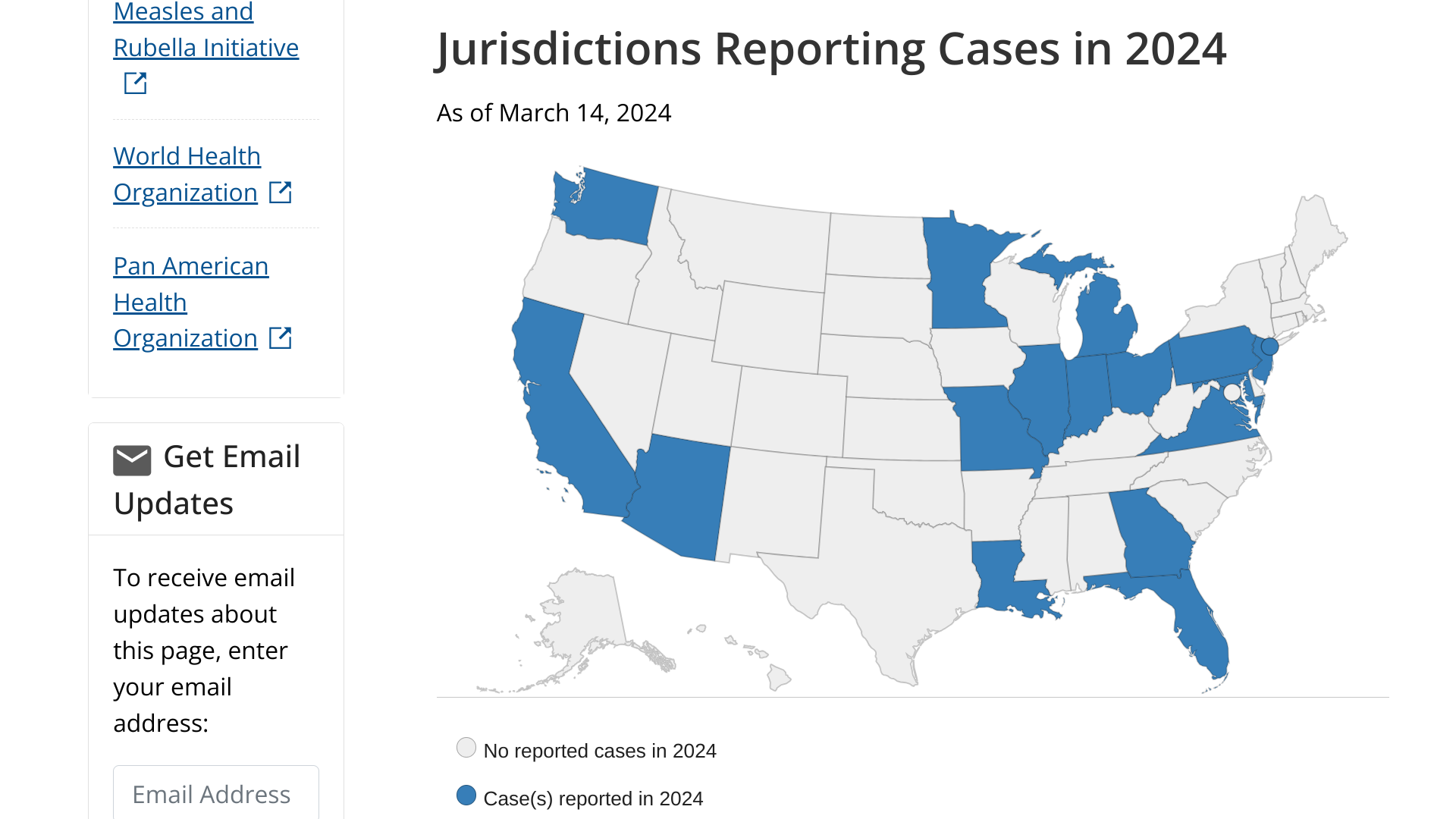
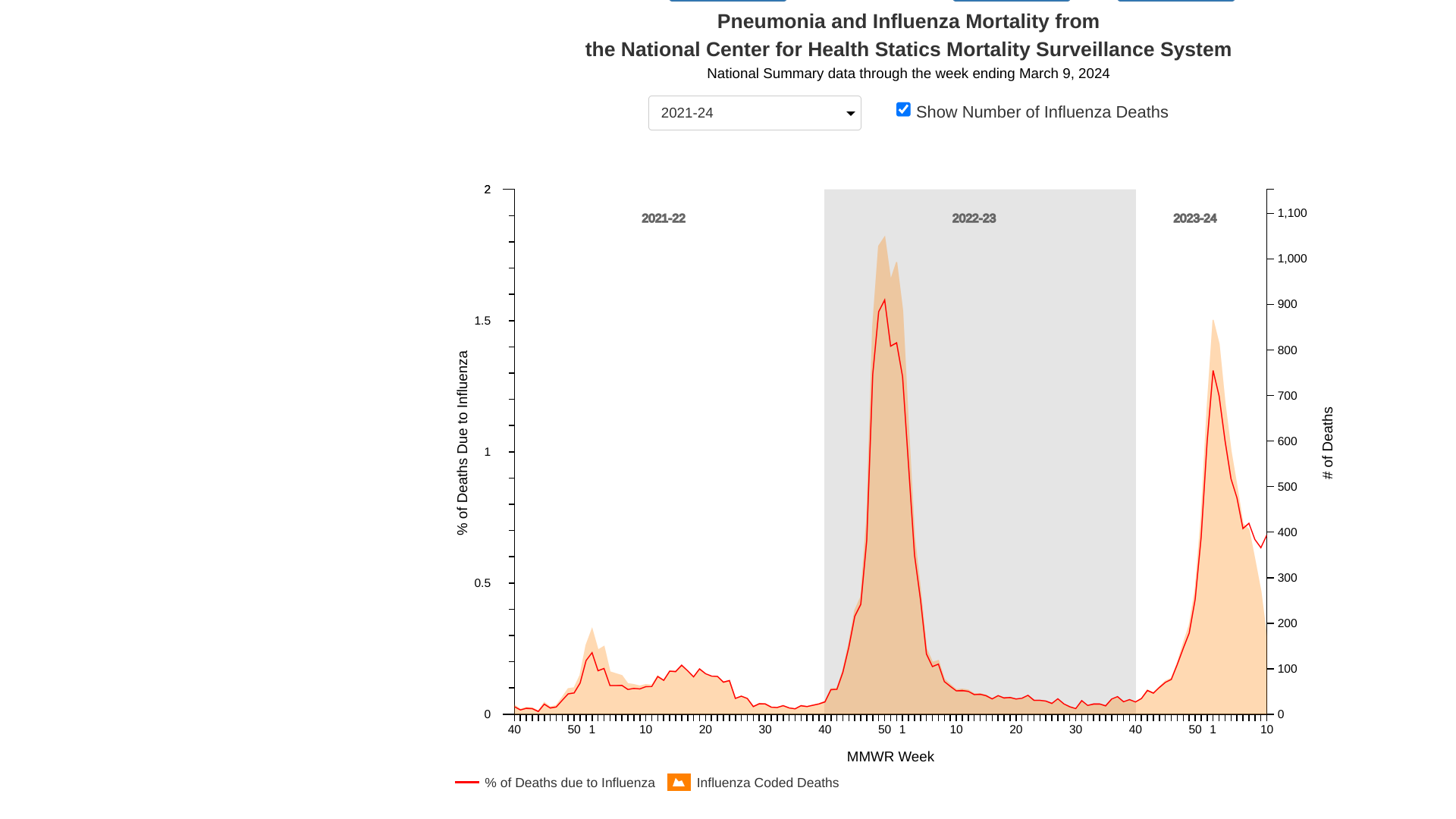
Virology Explain
Virology Explained
While various agencies approve drugs and vaccines, the related terms confuse most people. Virology's unanswered questions include:
When will precision vaccines replace population-based vaccination programs and transition to personalized vaccines, targeting each person's DNA?
How does immune imprinting impact vaccination benefits?
Do vaccines eliminate or enhance virus shedding?
Package Inserts
Government agencies issued broad media statements targeting the general public, and pharmaceutical companies disclosed what is known about a vaccine in U.S. FDA Package Inserts or U.S. CDC Vaccine Information Statements (VIS). These legal disclosures ensure clinical transparency but are difficult for most people to understand. Questions regarding vaccine coadministration, use while pregnant, and efficacy are best answered when speaking with your healthcare provider. Also, Vaccine Information Sheets are written to fulfill the National Childhood Vaccine Injury Act requirement; they do not replace Informed Consent.
Informed Consent
This FDA document is structured to present general guidance on FDA's regulatory requirements for informed consent. Furthermore, since there is no Federal requirement for Informed Consent relating to immunization, your state health department must fill this void. On December 21, 2023, the FDA issued a final rule providing an exception from the requirement to obtain informed consent when a clinical investigation poses minimal risk to people and includes appropriate safeguards to protect human subjects' rights, safety, and welfare. This rule permits institutional review boards to approve an informed consent procedure that waives or alters certain informed consent elements or waives the requirement to obtain informed consent under limited conditions, specifically FDA-regulated minimal-risk clinical investigations.
Drug Vaccine Interactions
Regarding adverse drug interactions, a study published by Pediatrics in 2024 estimates that 21% of U.S. children who take prescription drugs (vaccines) will experience a severe problem from a drug-drug interaction over a year of treatment, with the risk higher among those who take more than two drugs.
According to the U.S. CDC, adverse drug events cause approximately 1.3 million emergency department visits each year. Certain vaccines, including pneumococcal, meningococcal, and live virus vaccines, require appropriate spacing to mitigate vaccine–vaccine interactions.