Search API
The U.S. Centers for Disease Control and Prevention (CDC) has released its latest Weekly U.S. Influenza Surveillance Report for Week 5, confirming the 2025-2026 flu season activity remains elevated nationally.
However, patterns are shifting as the season progresses.
According to the CDC on February 13, 2026, influenza A activity is decreasing nationally and in most regions, while influenza B activity is increasing in many areas. Regional trends vary; some parts of the country are experiencing stable or declining levels, while others continue to see ongoing circulation of the virus.
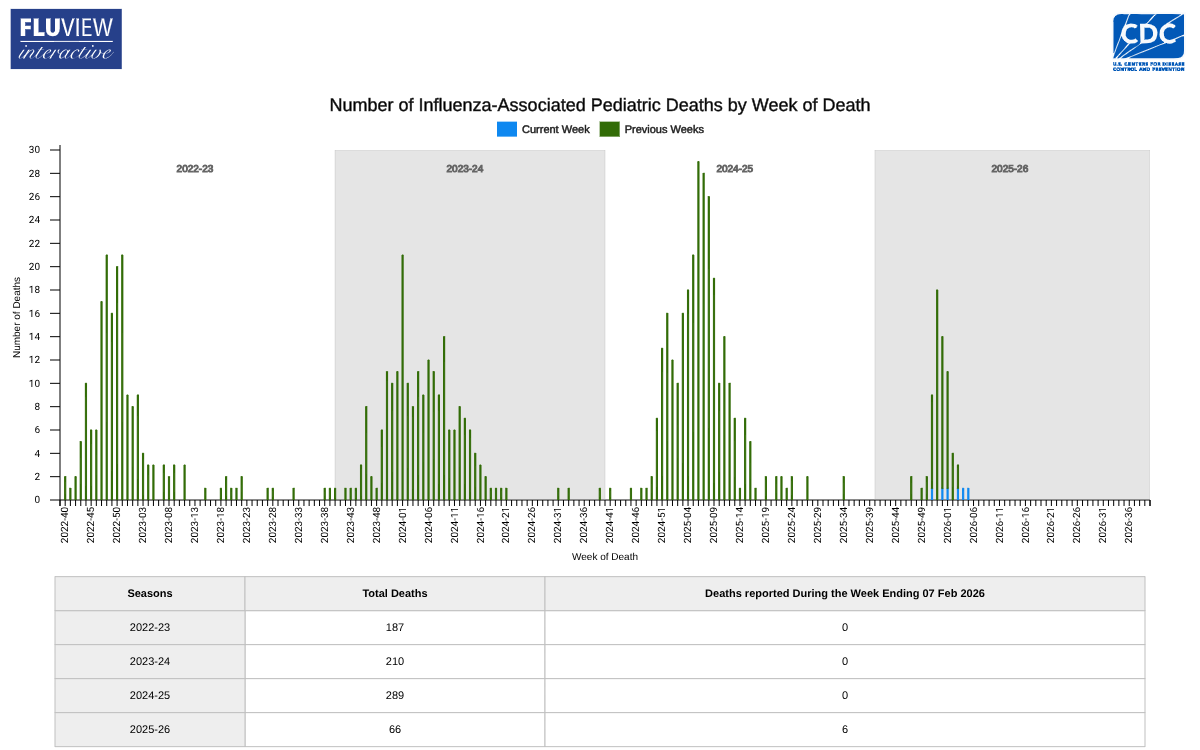
A significant concern raised in the report is the addition of six influenza-associated pediatric deaths recorded last week.
Four of the deaths were linked to influenza A viruses, with two cases identified as A(H3N2). The remaining two deaths were associated with influenza B viruses, although no lineage was determined.
This unfortunate news brings the cumulative total for the 2025-2026 season to 66 reported influenza-associated pediatric deaths.
Currently, precise state-by-state pediatric death locations or counts are not publicly disclosed by the CDC.
These totals compare with the last three full seasons: 2022-2023 season: 187 pediatric deaths reported; 2023-2024 season: 210 pediatric deaths reported; 2024-2025 season: 289 pediatric deaths reported, the highest for any non-pandemic season.
Among children eligible for vaccination who had known vaccination status, the CDC's data indicates that approximately 90% of these deaths occurred in those who were not fully vaccinated against influenza.
With about 130 flu shots already distributed in the U.S., access to vaccines such as FluMist continues in February 2026.
For the full CDC report and detailed surveillance data, visit the CDC's FluView website. Please note that all figures are preliminary and subject to updates as more reports are received.
The Regional Health Agency (ARS) of Réunion has recently confirmed a second imported case of smallpox (mpox) from the Republic of Madagascar to the French Department of Réunion.
As of February 10, 2026, the ARS reported that there is no established epidemiological link between this new case and the first case confirmed on January 22, 2026.
An investigation has been conducted to identify at-risk contacts in Réunion, who have been individually informed and directed to seek medical follow-up. This follow-up may include the prescription of a reactive vaccination as soon as possible.
The ARS reminds everyone that anyone with suggestive symptoms (skin rash, ulcers, fever, swollen lymph nodes, etc.), particularly travelers returning from Madagascar or an area with active virus circulation, should contact their doctor or the SAMU-Centre 15 immediately, and isolate themselves immediately while awaiting medical advice.
Furthermore, in accordance with national recommendations, preventive vaccination (JYNNEOS®, MVA-BN®) is offered to the most exposed people (travelers going to areas of active circulation, people with multiple sexual partners, sex workers, exposed health professionals, and immunocompromised people.
And reactive vaccination is offered to at-risk contacts of confirmed cases, ideally within 4 to 14 days after exposure.
Vaccinations are carried out at authorized vaccination centers, such as the North and South University Hospital and CEGIDD West.
Mpox clade Ib cases in Madagascar began in late 2025, marking the country's first documented outbreak. As of early February 2026, approximately 250 confirmed mpox cases had been reported, concentrated in the Boeny region.
This report concerns tens of thousands of travelers who visit this island off Africa's eastern coast each year.
Before visiting Madagascar in 2026, the U.S. CDC recommends that at-risk travelers receive their first mpox vaccine at least 6 weeks before travel, if possible. Numerious travel vaccine clinics in the U.S. offer mpox vaccination services.
For more than 100 years, the effectiveness of tuberculosis (TB) vaccines has varied based on the type of vaccine used.
As of 2026, there are over ten TB vaccines in circulation, prompting researchers to explore ways to enhance their effectiveness in decreasing the number of new cases.
Given the rising number of TB cases in the United States and other countries in 2026, this research is crucial for reducing the incidence of the disease.
According to a mathematical modeling study, the population-level success of new TB vaccines could hinge on their ability to block infectious asymptomatic TB, where people transmit the bacteria without symptoms.
Using models based on high-burden settings, researchers compared three vaccine scenarios: preventing progression to infectious symptomatic TB only; preventing progression to any infectious disease, including asymptomatic disease; and preventing progression to any disease.
Across all short-term (3-year) scenarios, symptomatic TB cases were reduced by a similar amount (≈1.6–2.3%).
Over longer periods (20 years), vaccines that block infectious asymptomatic disease averted far more cases—19.4% and 23.3% vs. just 7.3% in the symptomatic-only scenario—mainly by curbing silent transmission.
Published in PLOS Medicine on February 12, 2026, the study highlights that overlooking efficacy against asymptomatic infection could underestimate the long-term benefits of new TB vaccines in ending the global epidemic.
These researchers also evaluated scenarios in which they hypothetically assumed the vaccines would be effective in the pre-symptomatic stages at the time of vaccination.
If this were the case, they would have seen greater impact from the vaccines because they protected a larger proportion of the population, specifically those at high risk of progressing to later stages of disease.
However, experts believe that it is unlikely that a vaccine would be effective if delivered to someone with the disease at the time of vaccination, as the immune response would overwhelm any vaccine effect.
Although it is not yet known whether the same applies to earlier disease stages (such as nTB) and to undulation between disease stages, these researchers wrote.

The Pan American Health Organization (PAHO) issued an epidemiological alert on February 10, 2026, warning of a sustained rise in chikungunya cases across parts of the Americas.
The alert highlights increased transmission from late 2025 through early 2026, including the resumption of local transmission in territories that had not reported the virus for several years, including the United States.
While rarely fatal, the illness can cause debilitating long-term effects.
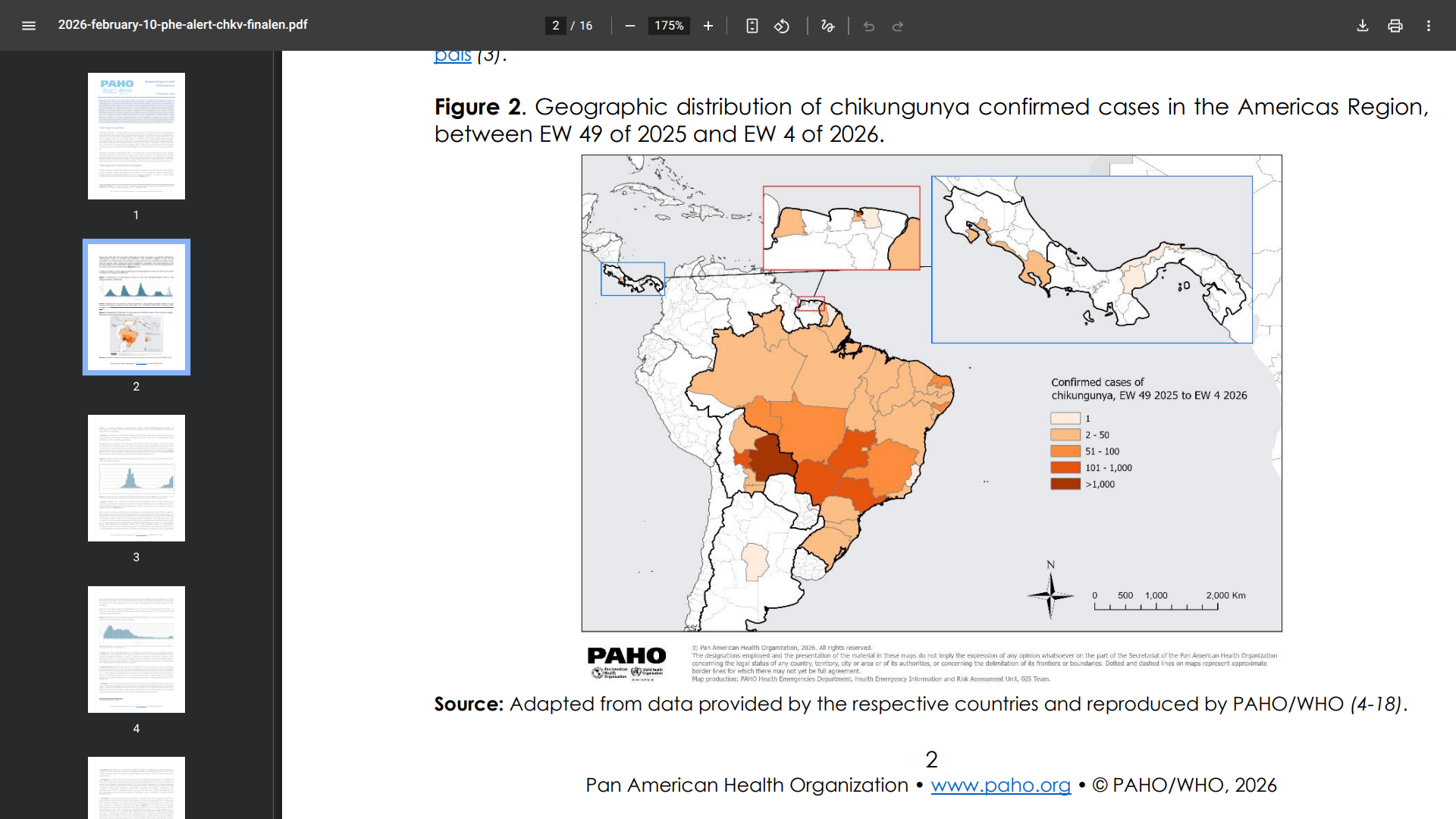
According to PAHO's alert, as of epidemiological week (EW) 4 in 2026, a total of 7,150 chikungunya cases had been reported, with 2,351 confirmed and 1 death recorded.
This uptick follows a broader regional trend: in 2025, the Americas reported 313,132 cases (113,926 confirmed, including 170 deaths) across 18 countries and one territory.
Notable activity occurred in Brazil's central-western and southeastern regions, southern Bolivia, and the re-emergence in the Guiana Shield area (including Guyana, French Guiana, and Suriname after nearly a decade without reports.
The PAHO noted that while dynamics may reflect cyclical epidemiology, the reappearance in previously quiet territories underscores the urgency for heightened vigilance.
Genomic analysis shows three main global genotypes of the chikungunya virus: West African, East/Central/South African, and Asian. Specifically, the Indian Ocean lineage, which features the E1-A226V mutation, enhances transmissibility, contributing to wider spread in some contexts.
Chikungunya is a viral disease transmitted primarily by Aedes aegypti and Aedes albopictus mosquitoes, the same vectors responsible for Dengue and Zika infections, which have also been reported in 2026.
In a positive development for prevention, approved chikungunya vaccines are expected to be available in 2026. These include virus-like particle options, such as the VIMKUNYA vaccine.
This vaccine is recommended for people visiting areas with chikungunya outbreaks and is commercially offered at certified travel clinics throughout the U.S.
With international travel forecast to increase again in 2026, a group of innovative vaccines is showing positive trends.
Bavarian Nordic A/S today announced its preliminary, unaudited financial results for 2025, highlighting strong demand for its travel vaccines.
On February 12, 2026, the company confirmed that its Travel Health business exceeded its latest financial guidance, reporting a 30% increase compared to 2024.
The demand for its existing rabies and Tick-Borne Encephalitis vaccines continued to grow, with increases of 34% and 20%, respectively.
Additionally, the first-year sales of the chikungunya vaccine (VIMKUNYA®) reached DKK 85 million (approximately $13.4 million).
While the mosquito-transmitted chikungunya virus has affected over 100 countries since its emergence, the state of Florida is reporting numerious travel-related cases and even an unusual local case in early 2026.
These increases exceed the global international travel activity, which only increased by 4% from 2024 to 2025, according to data from UN Tourism.
Paul Chaplin, President and CEO of Bavarian Nordic, commented in a press release, "We are encouraged by these extraordinary results."
We remain, however, the leading provider to governments of mpox and smallpox vaccines to support their public health responses, whether during outbreaks or for long-term stockpiling, and we continue to build strong partnerships to expand the base business."
The unedited company press release is found at this link.

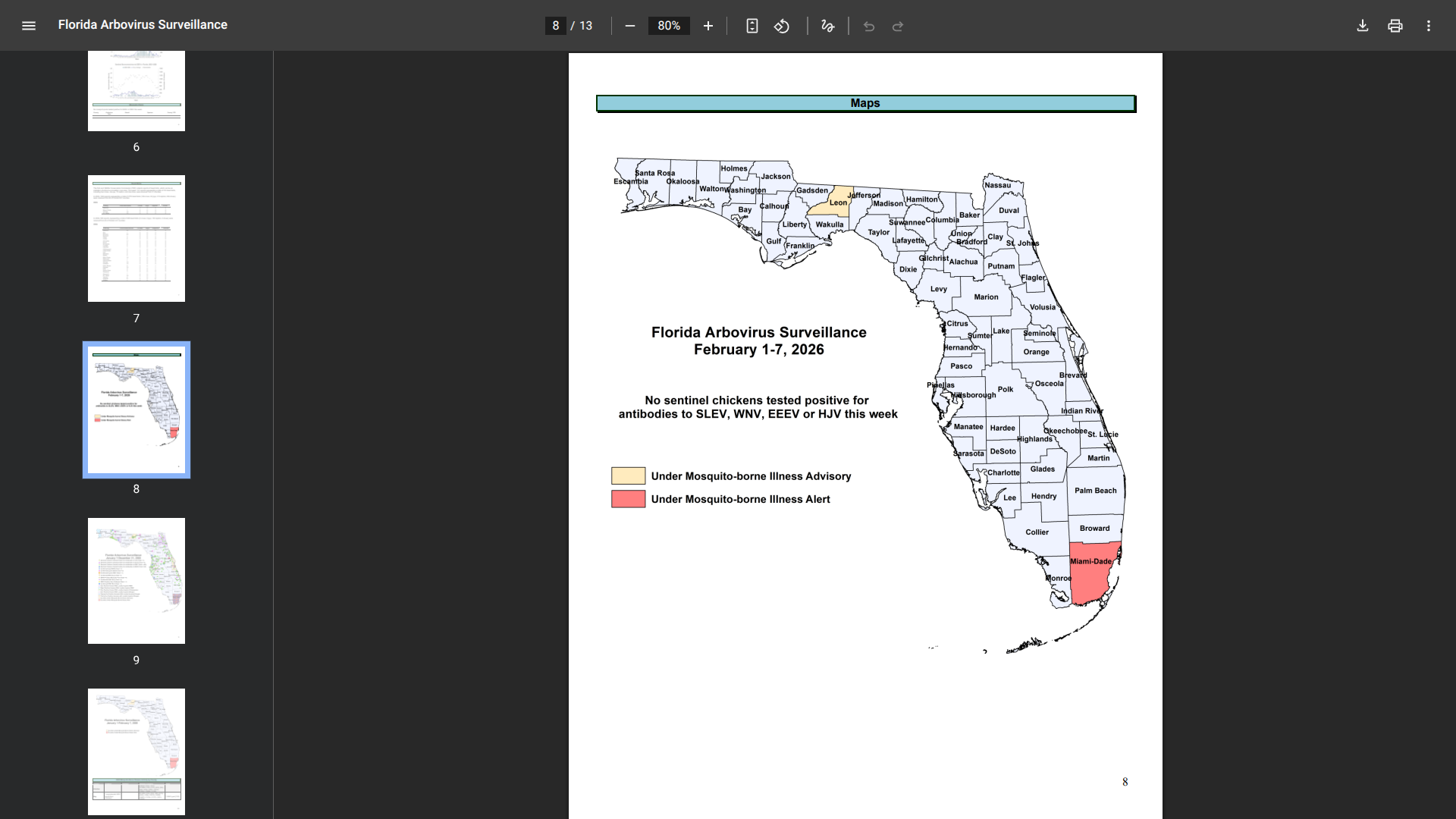
Health officials in Florida are monitoring a significant increase in chikungunya fever cases, predominantly linked to travel from Cuba, where the mosquito-transmitted virus was associated with more than 50,000 cases in 2025.
According to week #5 data from the Florida Department of Health (DOH), the state recorded a high number of travel-associated cases this year.
As of early February 2026, health officials have reported 16 cases with onset in 2026 among individuals with a recent travel history from Cuba. The most affected counties include Miami-Dade (10 cases) and Palm Beach (2 cases).
In 2025, Florida reported 370 travel-associated chikungunya cases. Of these, 357 were linked to travel to Cuba.
Furthermore, the DOH reported a rare but notable case of locally acquired chikungunya fever in Miami-Dade County, with symptom onset in December 2025.
This marks the first instance of local transmission in Florida since 2014, when 12 cases were reported.
Local transmission in Florida indicates the virus was passed from an infected traveler to a resident through mosquitoes within the state, raising concerns about the potential establishment of the virus in areas with competent mosquito vectors in 2026.
As of February 12, 2026, the U.S.CDC maintains a Level 2 Travel Health Notice ("Practice Enhanced Precautions") for chikungunya in Cuba, advising travelers to use insect repellent, wear long sleeves and pants, and take other measures to prevent mosquito bites.
Additionally, the CDC advises travelers visiting chikungunya-endemic areas to speak with a vaccine expert about the 2026 immunization option.
Hong Kong's Centre for Health Protection (CHP) reported two new human cases of H9N2 avian influenza infection from mainland China, with symptom onset in late December 2025 and mid-January 2026.
The announcement, published on February 10, 2026 (Week 6), highlighted ongoing sporadic detections of the low-pathogenic bird flu virus in humans.
These cases in Hubei, Guangxi, and Jiangsu provinces coincided with the reporting of an additional human infection involving avian influenza A(H10N3) in a 34-year-old man from Guangdong Province, whose symptoms also began on December 29, 2025.
If confirmed, this would mark only the seventh known human case of H10N3 globally.
According to CHP data, 20 H9N2 cases have been reported in China over the past six months.
Unlike highly pathogenic strains like H5N1, H9N2 has not shown sustained human-to-human transmission.
In the entire year of 2025, mainland China recorded 29 H9N2 cases, which marks a significant increase compared to previous years (11 cases in 2024).
While global human H9N2 infections remain relatively low, they have risen in recent years, primarily in China, with occasional reports from neighboring regions.
Since the first confirmed human case in 1998 (Hong Kong), officials stress that although H9N2 infections are uncommon in humans, continued surveillance is essential because the virus circulates in poultry and can reassort with other influenza strains.
H9N2 is a low-pathogenic avian influenza virus commonly found in poultry, particularly in live bird markets across parts of Asia. Human infections are generally mild, resembling the seasonal flu, with symptoms such as fever, cough, and sore throat; most patients recover fully without severe complications.
Another virus subtype is notable for causing severe illness in people, despite being classified as low pathogenic in birds. As previously reported, between March 2013 and September 2019, a total of 1,568 human cases of avian influenza A(H7N9) have been reported globally.
As of February 12, 2026, the CHP and the World Health Organization (WHO) (January 2026) say the overall public health risk from these cases is assessed as low. The WHO does not advise special traveller screening at points of entry or restrictions about the current situation of influenza viruses at the human-animal interface
Furthermore, these agencies say vaccination options are currently limited.