Search API
The Chicago Department of Public Health (CDPH) is currently monitoring a cluster of invasive meningococcal disease (IMD) cases.
Since January 15, 2026, ten adult cases and two related fatalities have been confirmed.
As of mid-February 2026, this Chicago outbreak is primarily affecting the West Side and Southwest Side of the city, with case numbers far exceeding the typical 0-2 cases reported in January over the past decade.
All tested isolates from this outbreak are identified as serogroup Y, which has been the dominant strain in Chicago since 2022.
CDPH recommends the MenACWY vaccination (Menveo, MenQuadfi) for individuals experiencing homelessness and encourages hospitals to vaccinate at-risk patients upon discharge.
Nationwide, IMD cases have been increasing sharply since 2021. In 2024, there were 503 confirmed and probable cases (based on preliminary data), marking the highest annual total since 2013.
According to CDPH, Neisseria meningitidis, the bacteria that cause IMD, is transmitted through direct contact with saliva. However, it typically requires close and prolonged contact to spread.
For the most accurate and up-to-date national figures, please visit the CDC's meningococcal disease surveillance webpage.

The Pan American Health Organization (PAHO) recently announced it has partnered with the Ministry of Health, Wellness, and the Environment of Saint Vincent and the Grenadines to promote the rollout of the human papillomavirus (HPV) vaccine for school-aged children.
On February 11, 2026, PAHO stated that HPV vaccination is a crucial component of cervical cancer prevention, as persistent infections with high-risk HPV types account for the majority of cervical cancer cases throughout the Caribbean Islands.
This collaboration is particularly important in Saint Vincent and the Grenadines, where cervical cancer poses a significant public health challenge.
Recent data revealed that 17 women were diagnosed with cervical cancer in 2024, marking an 88.9% increase from 2023. An additional 17 new cases were recorded in the first nine months of 2025.
From 2020 to 2024, a total of 44 women succumbed to this vaccine-preventable disease.
In 2023, cervical cancer accounted for 12.7% of all cancers among women in Saint Vincent.
The situation is similarly alarming across the Caribbean region, including Cuba and Puerto Rico, which have one of the highest rates of cervical HPV infection globally, at approximately 16% among women, ranking second only to sub-Saharan Africa.
Every year, more than 78,000 women in the Americas are diagnosed with cervical cancer, leading to over 40,000 deaths, 83% of which occur in Latin America and the Caribbean.
Dr. Amalia Del Riego, PAHO/WHO Representative for Barbados and the Eastern Caribbean Countries, welcomed the strong community response to this initiative. "We congratulate the Ministry of Health for advancing the elimination strategy by actively engaging communities," she stated.
"This partnership builds on recent milestones in the country, including the introduction of HPV DNA testing for screening (launched in September 2025) and expanded access to diagnostic and treatment services."
These actions, from vaccination to screening and treatment, represent a holistic approach to eliminating cervical cancer, in line with PAHO and WHO regional and global goals for 2026.
In a significant move to eliminate one of the world's last remaining polio-endemic threats, the Government of Japan has provided $6.3 million to support polio eradication and strengthen routine immunization services across all 34 provinces of Afghanistan.
This 12-month initiative, announced on February 11, 2026, will support the procurement and distribution of oral polio vaccines (OPV) to reach more than 12 million Afghan children.
The novel OPV (nOPV2) has been deployed about 2 billion times in recent years.
Afghanistan is one of only two countries, along with Pakistan, where wild poliovirus type 1 is still circulating, posing a serious risk of paralysis and death to unvaccinated children.
While significant progress has been made, with reported cases dropping sharply in recent years, transmission continues in the country's high-risk southern regions.
According to official data from the Global Polio Eradication Initiative and local health authorities, wild polio cases in Afghanistan decreased from 25 in 2024 to 13 in 2025.
As of early February 2026, no new wild polio cases have been reported in Afghanistan this year.
Globally, the total number of wild polio cases reached around 44 in 2025, comprising 13 in Afghanistan and 31 in Pakistan, a decline from 99 cases the previous year.
This trend highlights the positive impact of ongoing vaccination efforts.
This renewed financial support from Japan reinforces hope that Afghanistan will soon join the list of polio-free nations, thereby protecting future generations of children from this preventable disease.
A clinical-stage biotechnology company recently announced that the first participants have been dosed in the company's Phase 1A clinical trial evaluating Centi-Flu 01, a pan-influenza universal flu vaccine.
According to Centivax, the Phase 1A represents a key milestone toward a new kind of flu vaccine designed to provide broader, more reliable protection than standard seasonal vaccines, protecting against currently circulating strains, future strains, and pandemic strains.
The first data from this studyis expected within the year.
Unlike conventional seasonal influenza vaccines, which must be reformulated annually to attempt to match predicted circulating strains, Centi-Flu 01 is designed to focus both antibody and cellular immune responses on conserved regions of the influenza virus that cannot mutate and are shared across strains and distance subtypes.
This approach aims to generate broad, consistent, and durable immunity against both seasonal and pandemic influenza.
Sawsan Youssef, PhD, founder and Chief Science Officer of Centivax, stated in a press release on February 12, 2026, "A universal influenza vaccine allows us to be proactive—moving from annual guesswork to predictable, durable response."
In addition to safety, the study will evaluate efficacy based on established correlates of protection, using the gold-standard hemagglutination inhibition (HAI) assay against a panel of more than twenty flu strains—including currently circulating strains, historical mismatch strains, seasonal guidance strains, and pandemic strains—in a direct head-to-head comparison with existing standard-of-care flu vaccines.
Because the HAI assay is the same correlate-of-protection used to license seasonal flu vaccines, positive data will provide a clear benchmark demonstrating the candidate's ability to deliver broad protection with a single vaccine.
This type of innovative flu shot is essential because current vaccines are suboptimal at preventing virus transmission.
The 2024-2025 seasonal flu vaccine (trivalent formulation provided moderate protection in the United States, according to interim estimates from the U.S. CDC. Seasonal influenza effectiveness estimates among children and adolescents was 32%, 59%, and 60% in outpatient settings.
With over 2 million spectators expected to visit Italy during the Winter 2026 Olympic Games, this large gathering has not yet faced any health emergencies.
As of February 16, 2026, and following the latest update from the European Centre for Disease Prevention and Control (ECDC), no significant public health events related to communicable diseases have been identified in connection with the Winter Olympic Games.
The ECDC's Communicable Disease Threats Report for week #7 states that the probability of European citizens contracting communicable diseases during the Winter Olympic and Paralympic Games 2026 is low if general preventive measures are implemented.
Furthermore, there are no vaccination requirements to attend the Games.
However, the U.S. CDC recommends various routine and travel vaccinations before visiting Italy.
From a safety perspective, the U.S. State Department has issued a Level 2 Travel Advisory regarding civil unrest in Italy.
Should a health emergency arise, the U.S. Embassy in Italy says the consular sections in Milan, Rome, Florence, and Naples currently have reduced availability; however, the Consular Agency in Venice will be available to provide emergency American Citizen Services.
The U.S. government recommends that travelers enroll in the Smart Traveler Enrollment Program when visiting Italy. It is a free service that sends digital updates and alerts from U.S. embassies and consulates abroad.

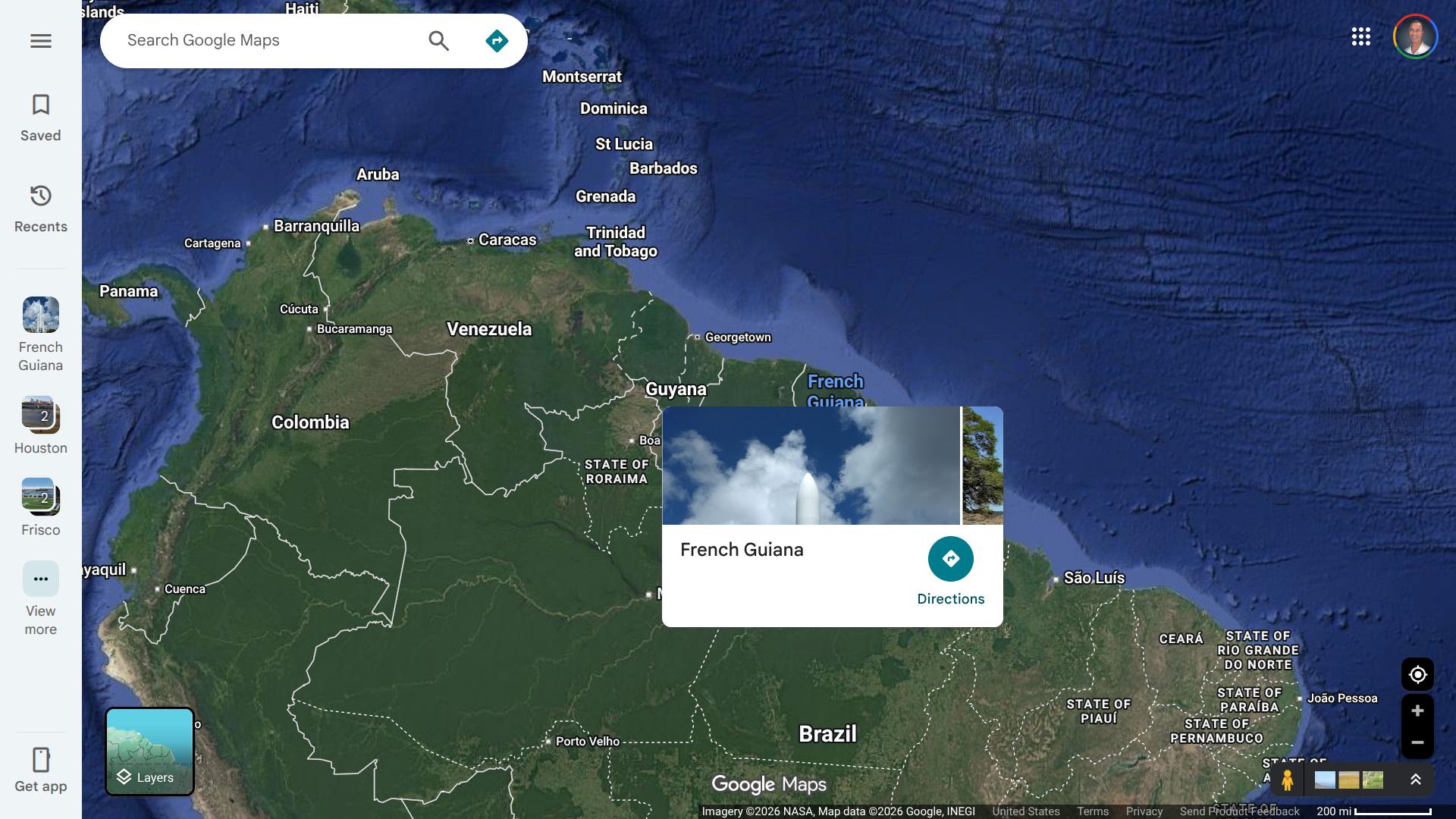
Health authorities in French Guiana, an overseas department of France located on the northeast coast of South America, have confirmed the re-emergence of local Chikungunya transmission for the first time since 2015.
The initial case was identified in a resident of Kourou by the French Guiana Regional Health Agency through RT-PCR testing on January 27, 2026.
This case was classified as locally acquired, meaning the patient contracted the virus from a mosquito bite in French Guiana, without having recently traveled to affected areas like Brazil. Subsequent investigations have revealed three additional confirmed cases.
The virus strain detected in these cases is genetically similar to the one currently circulating across parts of the Americas, according to the Pan American Health Organization (PAHO), which issued an epidemiological alert on February 10, 2026.
The alert noted the resumption of transmission in the Guiana Shield region—including Guyana, French Guiana, and Suriname.
The PAHO has urged heightened preparedness across the Americas amid rising chikungunya activity in several countries since late 2025. The reappearance of local transmission in areas long free of the Chikungunya virus underscores the risk posed by ongoing viral activity in neighboring countries and the potential for imported cases to spark outbreaks
This resurgence aligns with broader regional trends in 2025.
PAHO data indicate sustained increases in chikungunya cases in parts of South America and the Caribbean since late 2025, even as overall regional numbers have declined compared to the peaks in 2024.
The situation is under close monitoring by French health authorities and international partners, with no reports of severe complications or large-scale outbreaks at this stage.
However, in central South America, the U.S. Centers for Disease Control and Prevention (CDC) has identified parts of Bolivia as experiencing an ongoing chikungunya outbreak.
Currently, the CDC says there is no specific antiviral treatment; management focuses on symptom relief, and prevention relies on mosquito control and personal protective measures such as repellents, long clothing, and eliminating breeding sites.
Travelers to the region are encouraged by the PAHO and other agencies to consult health guidelines and consider protective measures, such as vaccination, before visiting the Guiana Shield region as of February 16, 2026.
South Korea recently confirmed its 15th case of African swine fever (ASF) for the season, prompting an escalation of quarantine efforts across the country during the Lunar New Year holiday to prevent further spread.
On February 14, 2026, the latest ASF infection was identified at a pig farm in Changnyeong County, South Gyeongsang Province, southeast of the capital city of Seoul.
This case caps a sharp rise in 2026 outbreaks, starting with the first in Gangwon Province, followed by detections in Gyeonggi, Jeollanam-do, Jeollabuk-do, Chungcheongnam-do, and multiple locations.
ASF is a highly contagious, often fatal viral disease exclusive to domestic pigs and wild boars, according to the U.S. Centers for Disease Control and Prevention (CDC).
As of 2026, ASF poses no threat to human health because it is non-zoonotic and cannot infect people through contact with pigs or by consuming properly cooked pork.
Even if someone consumes uncooked or undercooked pork from an infected pig, the virus does not infect human cells.
However, ASF outbreaks can cause substantial economic losses in the food supply.
First identified in 1921, the causative agent of ASF, African swine fever virus (ASFV), likely originated in a natural sylvatic cycle involving African wild suids and soft ticks, which serve as asymptomatic carriers and vectors, says the CDC.
A major global wave began in 2007 in Georgia with genotype II strains, reaching China in 2018 and triggering massive outbreaks across Asia and Europe.
In South Korea, ASF first appeared in domestic pigs in September 2019. The 2026 surge far exceeds the six farm outbreaks recorded in 2025.
The Ministry of Agriculture, Food and Rural Affairs has urged farmers and citizens in South Korea to avoid swill feeding, limit contact with wild boar, and prevent contaminated imports amid holiday travel.
As of February 2026, the USDA says ASF has never been detected in the continental United States.

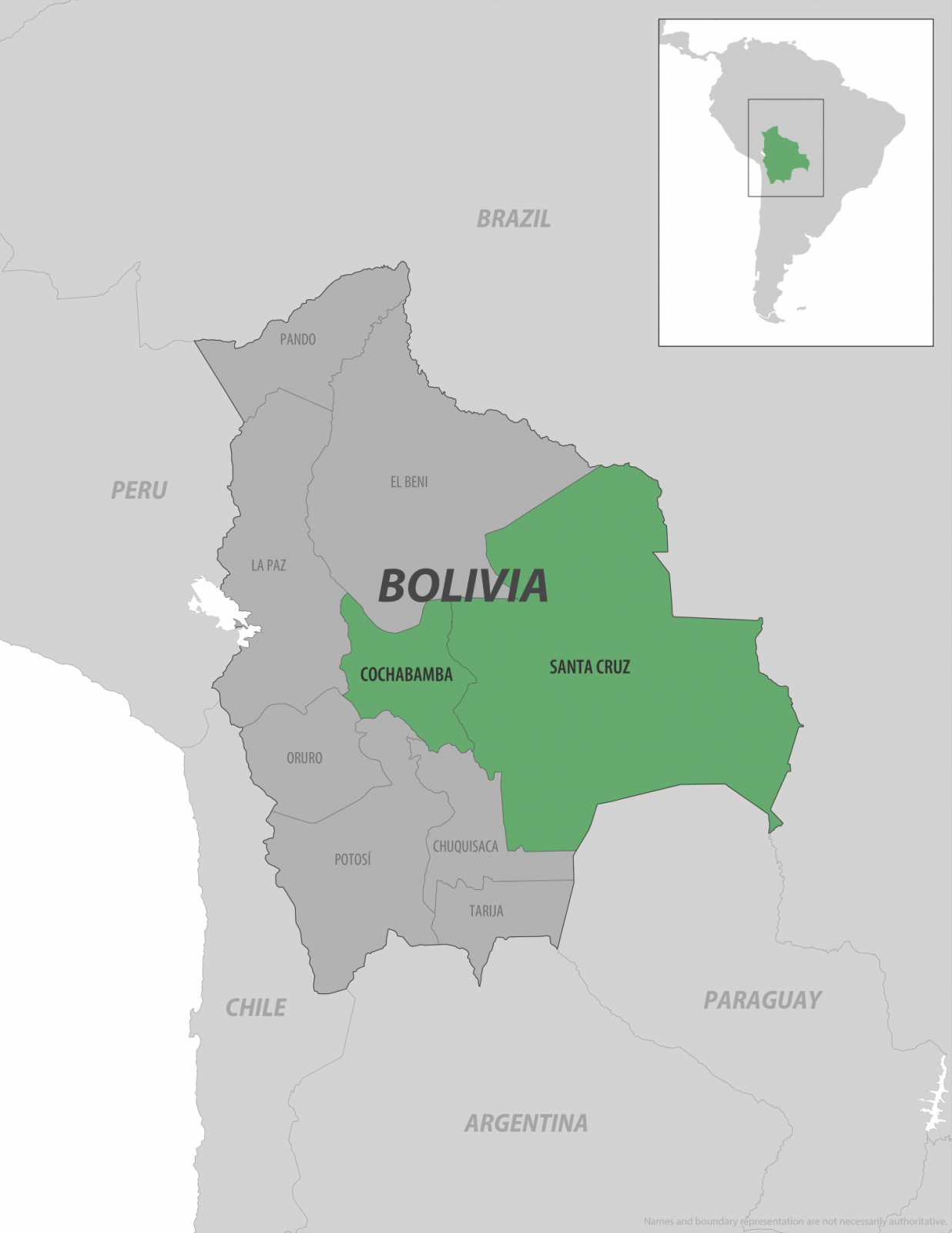
The U.S. Centers for Disease Control and Prevention (CDC) has identified parts of Bolivia as health risk destinations in South America due to an ongoing outbreak of chikungunya fever, a mosquito-borne viral illness.
As of mid-February 2026, there have been 1,534 confirmed or suspected cases reported nationwide.
A Travel Health Notice issued on February 11, 2026, classifies the situation as a Level 2 – Practice Enhanced Precautions, specifically highlighting outbreaks in the departments of Santa Cruz (eastern Bolivia) and Cochabamba (central Bolivia), while smaller numbers have been noted in Tarija (14), Beni (10), and Chuquisaca (7).
The CDC writes that Chikungunya, primarily transmitted by Aedes aegypti and Aedes albopictus mosquitoes, causes symptoms such as high fever, severe joint pain, muscle pain, headache, nausea, fatigue, and rash. While most individuals recover within a week, some may experience persistent joint pain for months or even years. Severe complications are rare but can occur, especially in vulnerable groups such as older adults, infants, and those with underlying health conditions.
There is no specific antiviral treatment available, but the CDC recommends a vaccine for travelers visiting outbreak areas.
In its notice, the CDC urges travelers to Bolivia, especially those visiting affected regions, to take proactive measures to avoid mosquito bites.
The outbreak in Bolivia is part of a broader regional trend in the Americas.
The Pan American Health Organization (PAHO) noted sustained increases in chikungunya cases since late 2025, with a re-emergence in areas such as the Guiana Shield (Guyana, French Guiana, Suriname) after nearly a decade without reports.
In 2025, the Americas reported 313,132 cases (113,926 confirmed, including 170 deaths) across 18 countries and one territory, though overall regional numbers declined compared to 2024.
The CDC and PAHO say travelers to outbreak areas should consult travel health providers about vaccination options before their trips and monitor updates, as conditions can evolve rapidly due to changes in mosquito populations and environmental factors.
To help reduce the number of Dengue fever infections, the Brazilian Ministry of Health recently launched a nationwide vaccination campaign targeting approximately 1.2 million frontline healthcare professionals.
The Butantan-DV vaccine is the world's first single-dose Dengue vaccine, developed by the Instituto Butantan in São Paulo. It is a third-generation vaccine that protects people against all four Dengue virus serotypes and is approved for individuals aged 12 to 59.
To kick off the campaign on February 9, 2026, 650,000 doses have been distributed nationwide, with more shipments planned. In Sergipe, the campaign is expected to benefit 18,200 healthcare professionals, with 7,900 doses.
With an investment of R$ 368 million ($70.5 million USD), the government has secured 3.9 million doses initially, with plans to increase production through partnerships, aiming to deliver up to 30 million doses in the second half of 2026.
Vaccination for the general population is expected to start later in 2026.
"Vaccination is starting with the entire multidisciplinary team registered with the Brazilian Public Health System. These are the people who knock on doors, visit people's homes, check for mosquito breeding grounds, provide follow-up care, and carry out mobilization efforts. They are also the professionals who are at the first point of contact when there are cases of Dengue fever," highlighted the Minister of Health, Alexandre Padilha, in a press release.
This initiative is essential, as Brazil has already reported more than 133,000 probable Dengue cases and 8 related fatalities in 2026.
In Europe, no Dengue cases have been reported in 2026, excluding the outermost regions, such as Martinique, Guadeloupe, and Réunion.
The primary way to combat mosquito-borne diseases such as Dengue, Chikungunya, and Zika remains the elimination of breeding grounds for the Aedes aegypti mosquito, as the U.S. CDC notes.
In addition to these diseases, the CDC has issued travel alerts for Measles and Oropouche outbreaks in Brazil.
As of February 16, 2026, the Butantan-DV vaccine is unavailable in the USA.