Search API
According to the World Health Organization, mpox outbreaks remain a global health risk. Since mpox is a vaccine-preventable disease, access to the approved vaccine is essential.
To address this need, Bavarian Nordic A/S, a global vaccine company, recently issued the following clarification regarding the Health Emergency Preparedness and Response Authority (HERA) framework agreement.
The initial order for 750,000 doses of the MVA-BN (JYNNEOS) smallpox/mpox vaccine announced on October 31, 2025, will be delivered in 2026 and is the result of a new joint procurement contract by the European Commission through HERA.
This represents the second order received in 2025, following the earlier award of a contract option from the U. S. Biomedical Advanced Research and Development Authority in the U.S. Department of Health and Human Services, announced in May.
Bavarian Nordic anticipates additional orders for MVA-BN over the course of the following year.
As of November 3, 2025, in the United States, the JYNNEOS vaccine is commercailly available at various health clinics and pharmacies.

The Public and Environmental Health Office at Colorado State University (CSU) has reported an unusual increase in pertussis cases this semester, with 14 confirmed cases in the fall 2025 semester.
According to a CSU media statement on October 30, 2025, pertussis, known as whooping cough, is a highly contagious bacterial infection that spreads through respiratory droplets when an infected person coughs or sneezes.
CSU students are encouraged to contact the Health Network's Immunizations Department to verify whether they are up to date with their TDAP vaccine. The health department emphasizes that vaccination is the best defense against this infection.
Located in Fort Collins, 65 miles north of Denver, a community of about 1 million, CSU has an enrollment of 33,000.
In Colorado, the TDAP vaccine is recommended by the Health Department for most students and residents.
Chikungunya is a viral disease transmitted to humans through infected mosquitoes. The first known outbreak occurred in 1952. In the past year, 100 countries have reported chikungunya outbreaks to the the European Center for Disease Prevention and Control (ECDC).
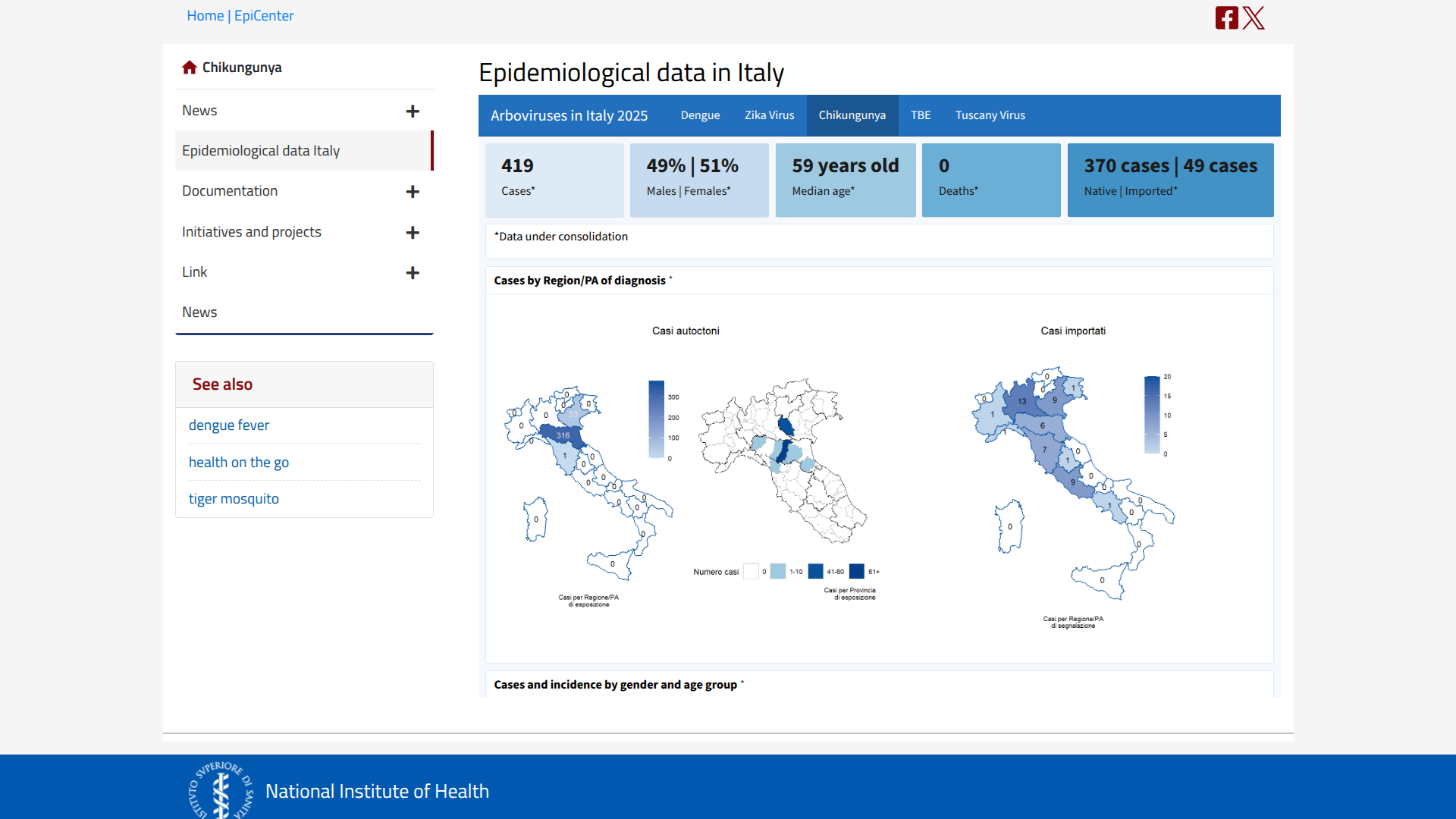
In Italy, the National Institute of Health has reported 419 chikungunya cases in 2025, with approximately 370 confirmed as locally transmitted, primarily in the northern region of the country.
According to ECDC's Week 44 report on October 29, 2025, the largest cluster of cases is located in Carpi, San Prospero, Soliera, Novellara, Cavezzo, Modena, Nonantola, Correggio, Novi di Modena, and Cesenatico.
In the United States, the CDC says locally acquired chikungunya cases have not been reported since 2019.
However, as of late November 2025, the CDC confirmed 19 travel-related cases in states such as Florida, several of which were linked to visitors from Cuba.
The CDC currently recommends that international travelers visiting outbreak areas speak with a travel vaccine provider about immunization options. Approved chikungunya vaccines will be available at clinics and pharmacies in November 2025.
A recent Oxford University-led study demonstrated that an oral live-attenuated vaccine, CVD 1902, provided significant protection against S. Paratyphi A infection in adults, without any safety concerns.
This phase 2b study is essential as enteric fever, caused by Salmonella Typhi and Salmonella Paratyphi, leads to more than 100,000 deaths and over 8 million disability-adjusted life years each year. Around 30% of cases —over 2 million annually —are caused by S. Paratyphi A, for which no vaccine is currently available.
According to the U.S. CDC, most people in the United States with these illnesses (Typhoid fever and paratyphoid fever) are infected while traveling internationally.
Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group and co-senior author, commented in a press release on October 30, 2025, "We are in a constant fight against bacterial infections, like paratyphoid, that threaten the lives of children in some of the most resource-poor regions of the world."
"This study provides hope that this important disease could be controlled by vaccination if the same effects can be obtained in real-life conditions in those communities."
The research was funded by the UK Medical Research Council and the National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre, with collaboration from Bharat Biotech International Ltd. and the University of Maryland, where the vaccine was originally engineered.
The paper, "Safety, Efficacy, and Immunogenicity of a Salmonella Paratyphi A Vaccine," by McCann et al., was published in The New England Journal of Medicine.

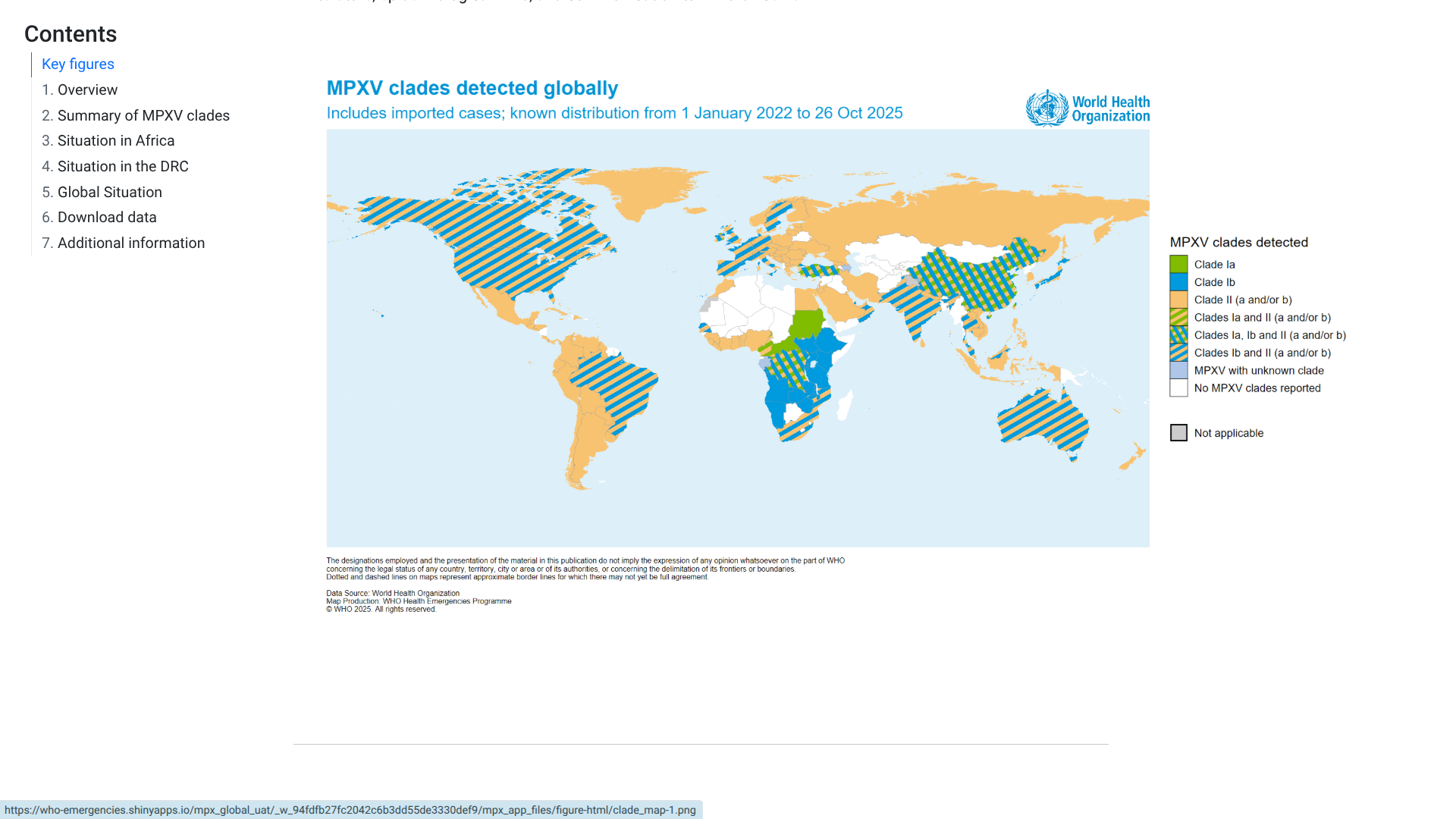
The World Health Organization (WHO) recently published the 59th situation report for the multi-country outbreak of mpox, which provides details on the global epidemiological situation for mpox.
As of November 2, 2025, the WHO stated all clades of the monkeypox virus (MPXV) continue to circulate across all regions.
In September 2025, 42 countries reported a total of 3,135 confirmed cases, including 12 related fatalities.
More than 80% of these cases were reported in the African Region.
The WHO wrote, 'In light of expanding community transmission of clade Ib MPXV and its detection among men who have sex with men, WHO currently assesses the public health risk as moderate for men who have sex with men and low for the general population in contexts outside historically endemic areas.'
From a prevention perspective, More than 684,000 doses of MVA-BN (JYNNEOS) and 118,000 doses of LC16 have been administered in the Democratic Republic of the Congo, which accounts for more than 61% of people vaccinated in African countries.
Other countries that recently reported mpox are developing national mpox vaccination plans and are encouraged to consider dose-sparing options of the MVA-BN vaccine.
Additional doses have been donated and procured, and partners continue to work together to support access to mpox vaccines and secure operational funds for implementation of national mpox vaccination plans.
In the United States, the JYNNEOS vaccine is offered at various health clinics and pharmacies and is recommended for at-risk individuals.
The Nipah virus, one of the world's deadliest viral pathogens, can lead to a variety of clinical outcomes in humans, ranging from asymptomatic infections to severe respiratory illnesses and fatal encephalitis, says the World Health Organization (WHO).
The WHO estimates its case fatality rate for this virus is between 40% and 75%.
During 2025, related fatalities were confirmed in Bangladesh and India.
Unfortunately, no preventive vaccines are available for this virus that is transmitted to humans from bats or pigs as of November 2, 2025.
Recently, a new defense strategy against the Nipah virus has been developed in collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI), the University of Oxford, and the Serum Institute of India Pvt Ltd., the world's largest vaccine manufacturer.
Their goal is to create the largest investigational-ready reserve of a Nipah virus vaccine candidate.
CEPI funding of up to $7.3 million will support SII for process development and manufacturing of the University of Oxford's ChAdOx1 NipahB vaccine candidate.
Dr. Umesh Shaligram, Executive Director, Serum Institute of India, commented in a press release in October 2025, "The collaboration with CEPI and the University of Oxford to develop and manufacture an investigational reserve of the ChAdOx-Nipah vaccine candidate marks a significant step forward in our pandemic preparedness efforts."
"Leveraging our proven manufacturing capabilities and past success with the ChAdOx platform, we are proud to help establish the world's largest investigational reserve against the Nipah virus—one of the deadliest pathogens known. Our aim is to ensure that life-saving doses reach those most in need, particularly across the Global South."
SII will manufacture doses for use in a Phase II clinical trial in a Nipah-affected country, and create an investigational reserve of up to 100,000 doses, which could be deployed under emergency use during a future Nipah virus outbreak, helping to generate critical data and potentially halt an epidemic in its tracks.
SII will supply the Phase II doses to the University of Oxford, which is conducting the clinical trials. These mid-stage trials are set to be the first Phase II trials for a Nipah virus vaccine candidate anywhere in the world.
Earlier in 2025, the CEPI and the U.S. Department of Defense launched a joint effort against Nipah virus treatment and prevention.
The Czech Republic (Czechia) is currently experiencing what may be the largest hepatitis A epidemic in the last 46 years, as reported by Ivana Lesková from MF DNES.
Since the arrival of the virus from Slovakia in April 2024, the State Institute of Health has documented 2,141 cases of hepatitis A and 26 related fatalities, including ten in Prague.
As of October 28, 2025, the Institute of Health has noted a significant increase in demand for hepatitis A vaccinations.
This year alone, more than 127,000 doses have been administered—almost double the total from last year. Due to this surge in demand, there are currently shortages of the vaccine.
However, the State Institute for Drug Control has announced to the local media that extraordinary vaccine shipments are on their way to the Czech Republic.
This vaccine shortage extends the HepA risk to over 20 million international travelers who may visit Czechia this year.
Currently, the U.S. CDC recommends that unvaccinated travelers aged 1 year or older visiting Czechia in 2025 speak with a healthcare provider about immunization options.
For international travelers who are allergic to a vaccine component, they should receive a single dose of immune globulin, which provides adequate protection for up to 2 months, depending on the dosage given.
Unvaccinated travelers who are over 40 years old, are immunocompromised, or have chronic medical conditions planning to depart for a risk area in less than 2 weeks should get the initial dose of vaccine and, at the same appointment, receive immune globulin, says the CDC.
According to the Pan American Health Organization (PAHO) mosquito-transmitted disease dashboard, Mexico has reported over 109,771 dengue fever cases in 2025.
As of October 27, 2025, there have been 48 dengue-related fatalities confirmed this year.
Last year, Mexico reported 558,846 dengue cases and 478 related fatalities.
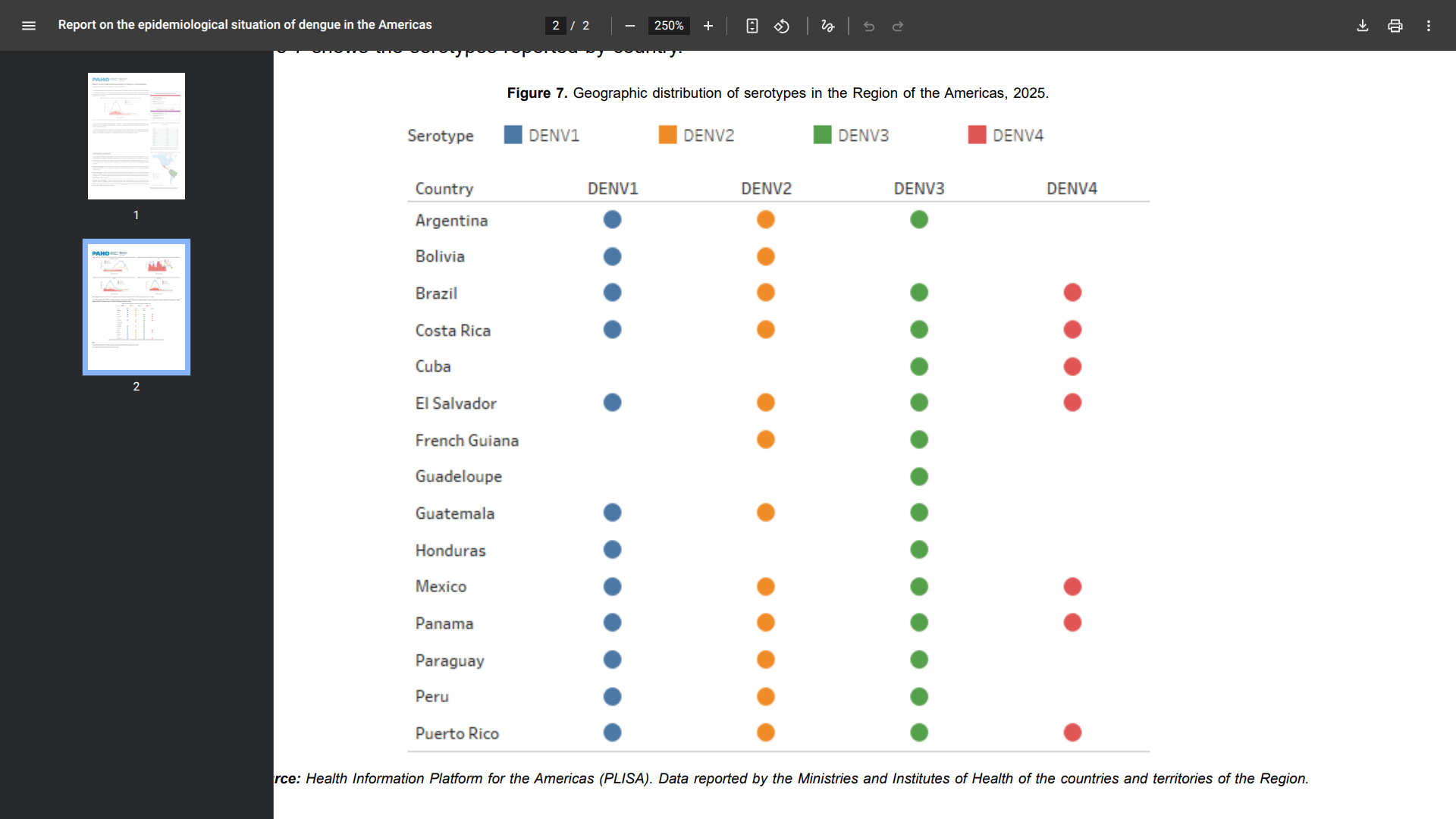
Specifically, during epidemiological week 39, 17 countries reported the circulation of dengue in the Region of the Americas: Brazil, Costa Rica, El Salvador, Mexico, and Panama. As of this week, the subregion exhibits a decrease of 77% compared to the same week in 2024
Several of these locations reported the the simultaneous circulation of DENV-1, DENV-2, DENV-3, and DENV-4 virus subtypes.
To Mexico's north, the Texas DSHS Arbovirus Activity Report Week #42 disclosed 45 dengue cases this year.
In 2024, Texas reported 143 dengue cases and one related fatality.
In Cameron County, one locally transmitted dengue case was confirmed.
Without access to dengue vaccines, Texas says, since Aedes mosquitoes usually bite people during the day, especially during early morning hours before daybreak and in the late afternoon before dark, when they are most active, use a mosquito repellent containing "DEET" on exposed skin to prevent infections.