Search API
GSK plc recently announced its MenABCWY vaccine PENMENVY could simplify meningococcal vaccination delivery and help protect more U.S. adolescents against these five common disease-causing serogroups – A, B, C, W, and Y, which commonly cause invasive meningococcal disease (IMD)/
To help realize that goal, the U.S. Food and Drug Administration has approved PENMENVY for use in individuals aged 10 through 25 years.
The vaccine combines the antigenic components of GSK's meningococcal vaccines, BEXSERO (Meningococcal Group B Vaccine) and MENVEO (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
Tony Wood, GSK's chief scientific officer, said in a February 14, 2025 press release, "We are excited about the opportunities ahead to help improve meningococcal vaccination coverage in the United States, especially for IMD caused by serogroup B."
"Building on our global leadership in meningococcal vaccination and our longstanding commitment to addressing unmet needs in disease prevention, we aim to help protect more teens and young adults at a life stage when they are at an increased risk."
A positive vote on PENMENVY's use is expected at the U.S. CDC's Advisory Committee on Immunization Practices meeting on February 26, 2025.

Zoetis recently announced it received a conditional license from the United States Department of Agriculture (USDA), Center for Veterinary Biologics, for its Avian Influenza Vaccine, H5N2 Subtype, Killed Virus for use in chickens, not people.
On February 14, 2025, the company wrote that the decision to vaccinate commercial poultry flocks against Highly Pathogenic Avian Influenza (HPAI) rests solely with national regulatory authorities in partnership with the poultry industry.
"When a new strain of HPAI was identified in the U.S. in early 2022, our scientists immediately began work to update our previous avian influenza vaccine," said Mahesh Kumar, Ph.D., senior vice president of global biologics research and development at Zoetis, in a press release.
"We first worked on HPAI vaccines in 2001-02 when outbreaks occurred in flocks in Southeast Asia."
"Our readiness with this most recent vaccine is another example of how we continue to live our purpose to nurture the world and humankind by advancing care for animals, ultimately providing solutions to global animal health challenges."
In 2016, the company received a conditional license for its H5N1 vaccine and a contract award for the USDA's National Veterinary Stockpile; this vaccine was first used by the U.S. Fish & Wildlife Service in 2023 to help protect California condors. Zoetis also holds a USDA license used to help protect endangered birds in New Zealand in 2024.
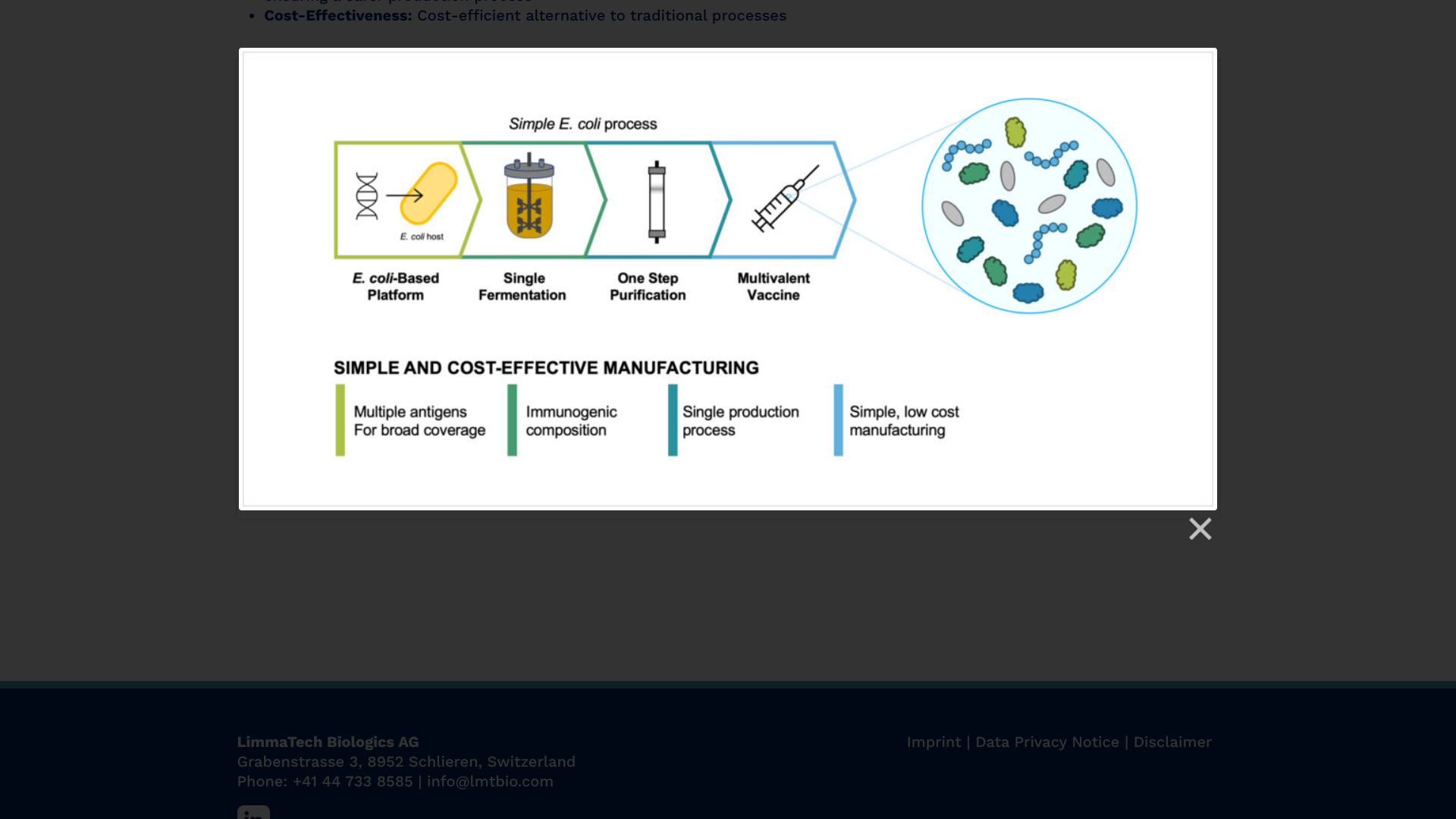
The first participants have been vaccinated in a Phase 1 clinical study of a multivalent vaccine candidate designed to prevent skin and soft tissue infections (SSTIs) caused by the bacterial pathogen Staphylococcus aureus (S. aureus).
S. aureus infections pose a significant global health challenge, causing an estimated 1 million deaths annually. Notably, 90% of all community-acquired S. aureus infections are SSTIs.
Furthermore, the World Health Organization has designated S. aureus a "high priority" pathogen, underscoring the urgency of developing innovative vaccine approaches and effective treatment strategies.
To address this essential health need, LimmaTech Biologics AG today announced that its LBT-SA7 vaccine candidate is expected to enroll 130 healthy adults aged 18-50 years, with initial results anticipated in the second half of 2025.
The company also announced the award of $6.5 million from the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) to advance the clinical development of LBT-SA7.
"Developing an S. aureus vaccine has long been a significant scientific challenge," explained Dr. Patricia Martin-Killias, Chief Operating Officer of LimmaTech, in a press release on February 17, 2025.
"We believe LBT-SA7 has the potential to provide a much-needed solution for those suffering from S. aureus infections. We are excited to launch the first-in-human clinical trial for LBT-SA7, bringing us closer to addressing an urgent global health challenge."
S. aureus is a Gram-positive bacterial pathogen that affects approximately 30% of the human population and causes a spectrum of infections, from SSTIs to severe conditions like pneumonia and bloodstream infections.
The company says traditional antibiotic treatments, both oral therapy and intravenous administration reserved for severe cases, have become increasingly less effective due to the rise of antibiotic resistance.
Federal funds from the U.S. Department of Health and Human Services partly fund CARB-X's funding for this project.
As the 2025 Daytona 500 NASCAR Cup Series starts-off today at Daytona International Speedway, over 150,000 people will be in attendance, many arriving by air.
The U.S. Transportation Security Administration (TSA) expects heavy travel volume from Daytona Beach International Airport (DAB) after the 500-mile race. The average daily passenger volume at DAB, around 1,000 passengers per day, will grow to an expected 2,200 on February 17, 2025.
"Planning is critical when traveling home after large events like this," said Brian Cahill, TSA Federal Security Director for DAB, in a press release. "Arriving at the airport with extra time and knowing what can and can't be packed in carry-on and checked bags will save you time and help keep things moving at checkpoints."
The good news for these travelers is that no health alerts have been issued for this area of central Florida.
The Volusia County Health Department has not issued any disease advisories this year.
However, travel-related and local diseases such as chikungunya, dengue, malaria, and Oropouche may impact race fans arriving from Miami-Dade County, located about 250 miles south along the east coast. Over the past few years, Florida's southeast coast has been a hot spot for mosquito-transmitted diseases.
As of February 2025, the U.S. CDC recommends two disease-prevention options: avoid mosquito bites and speak with a travel vaccine expert about U.S. FDA-approved immunization options. The CDC has issued Travel Health Advisories for numerous countries, but no alerts have been issued for the greater Miami area.
The initial Japanese encephalitis virus (JEV) case during the summer of 2025 in Australia's New South Wales (NSW) was likely acquired in the Murrumbidgee region. A previous case was identified in a resident of northern Victoria in January 2025.
On February 15, 2025, NSW Health's Executive Director of Health Protection, Dr. Jeremy McAnulty, said this recent case, which is currently recovering in hospital, will likely have acquired the infection in late December (2024) or early January while on holiday.
"This case, along with recent detections in pigs and mosquitoes in NSW and detections in Victoria and Queensland, highlights the risk of JE virus infection in a large stretch of NSW west of the Great Dividing Range," Dr McAnulty said in a press release.
"It is essential for people who live in or travel to these areas to be aware of the elevated risk and to take precautions against mosquito bites."
"Importantly, there is a safe, effective, and free vaccine to protect against JE which is available to anyone who lives or routinely works in various inland LGAs as well as for people who work in some other high-risk occupations."
In Australia, Valneva SE's IXIARO® —JESPECT® JEV vaccine is available through local General Practitioners, Aboriginal health services, and pharmacists. In 2025, this vaccine will be commercially offered at travel clinics and pharmacies in the United States.
As of February 16, 2025, JEV is the leading cause of viral encephalitis in twenty-four countries in the WHO South-East Asia and Western Pacific Regions, exposing more than 3 billion people to infection risks.
The Quezon City Government, through the City Health Department (QCHD), declared a dengue outbreak today as cases and related fatalities have surged in the city.
From January 1 to February 14, 2025, the City Epidemiology and Surveillance Division of QCHD recorded 1,769 dengue cases, nearly 200% higher than last year. Ten citizens, including eight minors, have already died from the mosquito-transmitted disease.
Fifty-eight percent of the reported cases involve school-aged children (5 to 17 years old).
Mayor Joy Belmonte has mobilized all assets and resources and ensured that programs and services are established and accessible for QCitizens to curb the outbreak.
“Our declaration of a dengue outbreak ensures that we are on top of the situation, and we are doing everything we can to protect our residents from this deadly disease, especially our children,” Mayor Joy Belmonte said in a press release on February 15, 2025.
Quezon City, a tourist top destination located northeast of Manila, is the most populous city in the Republic of the Philippines, with a population of about 2.9 million.
To alert international travelers of their health risks when visiting the Philippines, the U.S. CDC includes this Southeast Asia country in its Global Travel Health Advisory. Other disease risks in 2025 included chikungunya and measles.
Over the past few months, the positive reduction in tuberculosis (TB), the world's leading infectious disease killer, has reversed course in India. Local media has reported that Maharashtra health workers have reported over 24,000 cases as part of the ongoing 100-day campaign under the National TB Elimination Programme (NTEP).
On February 10, 2025, Dr Sandeep Sangle, the Joint Director of Health in Maharashtra, told The Indian Express, "The main aim is to enhance TB detection, improve the efficiency of TB testing and treatment, reduce the mortality rate, and prevent new cases through targeted interventions in the districts."
Previously, the NTEP reported the incidence of TB had been reduced from 237 per 1,00,000 population in 2015 to 195 in 2023, and the mortality rate has decreased from 28 to 22 in the same period.
The World Health Organization (WHO) issued an updated Global TB Report in 2024, revealing that approximately 8.2 million people were newly diagnosed with TB in 2023, the highest number ever recorded by the WHO.
In other United States, TB cases have steadily increased over the past three years. The U.S. CDC reported 8,040 TB cases in 2024, led by California (1,623), New York (901), and Texas (728).
TB is a vaccine-preventable disease, with various versions of the 100-year-old BCG vaccine offered globally.
While the CDC does not list TB in its Travel Health Advisories for India, it includes chikungunya, dengue, and Zika diseases. Of these diseases, the single-dose IXCHIQ® chikungunya vaccine is offered in travel clinics and pharmacies in the U.S., U.K., and Europe in 2025.
A recent study confirms that human papillomavirus (HPV) vaccination prevents the development of invasive cervical cancer, regardless of dosage.
Published in the Journal of the National Cancer Institute on January 22, 2025, this analysis concluded that even one or two doses one month apart confer benefit if given at 12-13 years of age. At older ages, three HPV doses are required for statistically significant vaccine effectiveness.
No cases of invasive cancer were recorded in women immunized at 12 or 13 years of age, irrespective of the number of doses.
Women vaccinated at 14 to 22 years of age and given three doses of the bivalent vaccine showed a significant reduction in incidence compared with all unvaccinated women (3.2/100 000 [95% confidence interval (CI) = 2.1 to 4.6] vs. 8.4 [95% CI = 7.2 to 9.6]).
Unadjusted incidence was significantly higher in women from most deprived (Scottish Index of Multiple Deprivation 1) than least deprived (Scottish Index of Multiple Deprivation 5) areas (10.1/100 000 [95% CI = 7.8 to 12.8] vs 3.9 [95% CI = 2.6 to 5.7]).
Women from the most deprived areas showed a significant reduction in incidence following three doses of vaccine (13.1/100 000 [95% CI = 9.95 to 16.9] vs 2.29 [95% CI = 0.62 to 5.86]).
Dr. Kirsty Roy, Consultant in Health Protection, Public Health Scotland, and co-author of this encouraging study, said in a press release, "This study involves every woman in Scotland who is eligible for the cervical cancer screening program and demonstrates the impact of the HPV vaccine in preventing cervical cancer."
"It shows how effective the HPV vaccine is as there have been no cervical cancer cases to date in fully vaccinated women who were given their first dose at age 12-13 years.
"Vaccination against HPV is shown to be effective in preventing cervical cancer, and along with regular screening for early detection and treatment, it is possible to make cervical cancer a rare disease."
In the United States, Merck's GARDASIL 9® vaccine has been found to protect women and men ages 9 to 45 against diseases caused by nine types of HPV. GARDSIL is generally available at clinics and pharmacies in the U.S.