Search API
As the summer vacation season 2025 gets underway, the U.S. Centers for Disease Control and Prevention (CDC) cruise ship illness inspections are on pace to set new, unfortunate records.
As of May 5, 2025, the CDC's Vessel Sanitation Program had reported 16 gastrointestinal (GI) illnesses on cruise ships this year, 14 of which were classified as norovirus.
This data compares with 18 GI outbreaks in all of 2024 and just 14 in 2023.
The CDC says traveling on cruise ships exposes people to new environments and thousands of other voyagers. Health risks come from contaminated food or water or, more commonly, through person-to-person contact and a lack of hand-washing.
The most recent report was from the cruise ship Eurodam. As of April 28, 2025, the final case counts were:
Number of passengers who reported being ill during the voyage: 148 of 2,038 (7.26%),
Number of crew who reported being ill during the journey: 22 of 830 (2.65%),
The predominant symptoms were diarrhea, vomiting, and abdominal cramps, and the causative agent was norovirus.
The CDC offers this quick tip to help about 20 million people vacationing on a North American cruise ship in 2025: To avoid getting sick, wash your hands often, especially after visiting a public bathroom and before/after eating a group-served meal.
With Disney soon to cruise to the western Caribbean from Galveston, Texas, more people should follow this CDC advice for the years ahead.
While norovirus vaccine candidates are being tested in clinical research to help prevent outbreaks, none are available in 2025.

Over the past Respiratory syncytial virus (RSV) seasons, infants have been offered a new monoclonal antibody to protect them from lower respiratory tract infections (LRTIs).
A cost-effectiveness analysis, published on May 3, 2025, estimates the economic benefits.
From the NHS perspective, over the first RSV season, Beyfortus™ (nirsevimab) in an all-infants population could be a cost-effective approach to preventing LRTIs.
These researchers concluded that a prophylaxis strategy against RSV infection targeting all infants with Beyfortus could represent a cost-effective option and support the implementation and equity of RSV prevention for all infants.
For the 2025-2026 RSV season in the United States, Beyfortus is FDA-approved and readily available.

The only protein-based, non-mRNA COVID-19 vaccine produced in the United States will continue to be available in Japan, the world's third-largest pharmaceutical market.
Novavax, Inc., announced on May 5, 2025, that it has updated the terms of the previously announced collaboration and licensing agreement with Takeda, a pharmaceutical firm located in Osaka, Japan.
"Our ongoing partnership with Takeda is important for Novavax, and our strengthened agreement enhances our ability to operate effectively in the Japanese market," said John C. Jacobs, President and CEO of Novavax, in a press release.
"This partnership further validates our cutting-edge technology platform and proprietary Matrix-M® adjuvant and our efforts to become a partner of choice."
The improved financial terms of the amended agreement for the development, manufacturing, and commercialization of Nuvaxovid® in Japan include an upfront payment, payment related to the 2024/2025 season, annual milestones associated with regulatory approvals, and royalties on net sales earned every season moving forward.
Since the World Health Organization granted an Emergency Use Listing for Novavax's earlier COVID-19 vaccine version in December 2021, numerous countries, including the United States, have authorized its use.
Novavax's vaccines are genetically engineered using three-dimensional nanostructures of recombinant proteins critical to disease pathogenesis.

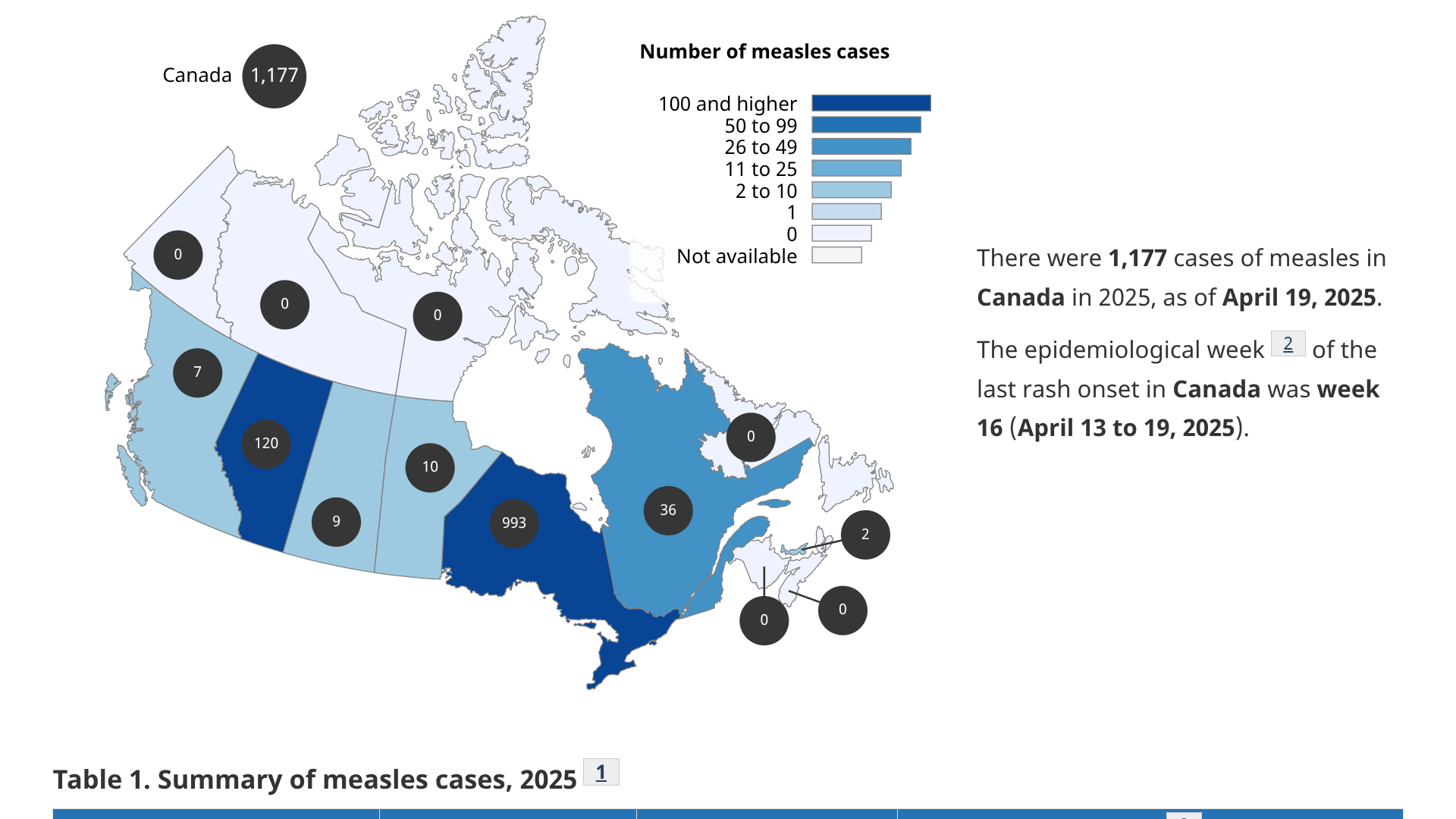
A multijurisdictional measles outbreak is ongoing in Canada and the United States in 2025.
Although measles was eliminated in Canada in 1998, an average of 91 cases, many travel-related, were reported annually.
According to the Canadian Government's data release on May 2, 2025, the current outbreak began in New Brunswick in October 2024 and has continued to spread in Alberta, Manitoba, Ontario, Prince Edward Island, and Quebec.
The data revealed 970 measles cases had been reported in Ontario, out of the 1,177 total cases in Canada. Of the virus genotypes analyzed, D8 was dominant.
During Ontario's outbreak, 451 measles cases have been confirmed in Ontario's Southwestern Public Health, including the City of St. Thomas, Elgin County, and Oxford County.
Data from Toronto, a city with over 2.7 million people, has confirmed only three cases.
These data indicate that Ontario's measles outbreak is primarily a rural, not urban, concern.
As of May 1, 2025, the Centers for Disease Control and Prevention (CDC) reported 935 confirmed measles cases in 30 U.S. jurisdictions, with Texas the leader.
Measles is an exceptionally infectious virus but can be easily prevented with an effective vaccine.
A study published by The Lancet in May 2025 supports using the MR vaccine at six months to protect young infants during measles outbreaks and in settings with increased risk or high transmission.
The CDC recommends evaluating your family's need for early vaccine doses before international travel. After you return, monitor your health for three weeks and call your healthcare provider if you or your child gets sick with a rash and fever.
The Republic of Indonesia's tourism sector experienced significant growth in 2024, with foreign tourist arrivals increasing by 20% compared to the same period in 2023.
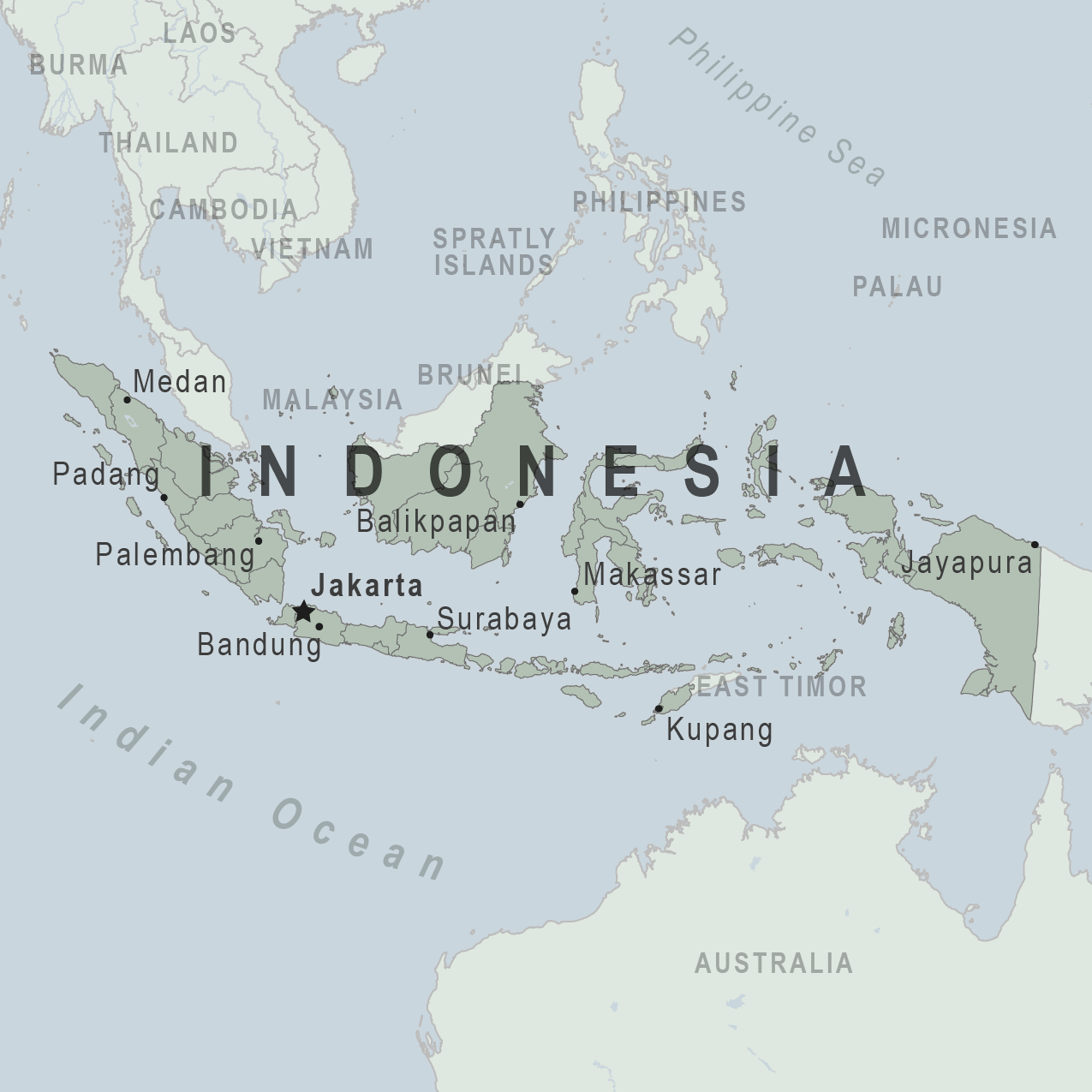
However, the U.S. Department of State recently issued a high-level advisory for international visitors to consider before visiting Indonesia in 2025.
On April 30, 2025, the U.S. Department of State advised visitors to exercise increased caution in Indonesia due to civil unrest.
Especially when visiting Central Papua and Highland Papua.
The Level 4, Do Not Travel advisory says that in Central Papua and Highland Papua, violent demonstrations and conflict could result in injury to U.S. citizens and foreign nationals. Due to the risks, the U.S. government cannot provide emergency services to U.S. citizens in Central Papua and Highland Papua.
Seperately, the U.S. CDC includes Indonesia in recent measles and polio travel advisories.
Additionally, there has been recent evidence of the transmission of the chikungunya, dengue, and Japanese encephalitis viruses in Indonesia.
The CDC recommends that everyone visiting Indonesia be up to date with routine vaccinations and consult with a vaccine expert to determine which travel vaccines are best suited for their visit.
The UK Health Security Agency (UKHSA) recently reviewed its guidance for countries at risk of dengue fever outbreaks. Last year, records of dengue outbreaks and related fatalities were set in many countries.
As of May 2, 2025, based on this review, most countries with a known risk of dengue now have a vaccine recommendation for some travellers.
However, the following countries have sporadic local dengue cases, but a vaccine is not recommended: Australia, Croatia, France, Italy, Madeira, Spain, the United Arab Emirates, and the United States.
In the U.S., locally acquired dengue was reported in 2025 in areas such as Miami-Dade County in Florida.
According to the UKHSA, health professionals should consider the possibility of dengue in all returned UK travellers with a fever or flu-like illness who have recently visited dengue risk regions. Suspected dengue patients should discuss this matter with their local microbiology, virology, or infectious diseases consultant, giving a full travel/clinical history.
The live, attenuated dengue vaccine called Qdenga® is licensed in the UK, but is unsuitable for all travellers.
Qdenga is currently not licensed in the U.S.
The UKHSA and NaTHNaC dengue recommendations are based on published data focusing on evidence of local mosquito-borne dengue transmission from January 2020 to December 2024.