Search API
India has retrospectively reported ten cases of mpox due to clade Ib mpox virus detected between December 2024 and March 2025.
According to the WHO's Multi-country outbreak of mpox, External situation report #52, as of May 14, 2025, all clade 1b cases reported in India had a recent history of travel to countries in the Gulf or contact with travellers from those countries.
Outside Africa, 16 countries have reported travel-related cases of mpox due to clade Ib: the United Kingdom of Great Britain and Northern Ireland (12 cases), Germany (10 cases), China (seven cases), Belgium (five cases), Qatar (five cases), Thailand (five cases), the United States of America (four cases).
Globally, Cases of mpox due to clade Ib continue to be reported primarily in Africa, where ten countries have reported community transmission of this strain, reaching over 14,000 cases in 2025.
Reported #52 discloses that mpox vaccination in countries in the African Region has more than 668,000 doses of MVA-BN (Bavarian Nordic A/S JYNNEOS®) vaccines administered in seven countries.
From the total number of JYNNEOS doses, 87% have been administered in the Democratic Republic of the Congo.
In the United States, the JYNNEOS vaccine is commercially available at many pharmacies.
The U.S. Food and Drug Administration (FDA) initially approved JYNNEOS for smallpox prevention in September 2019. And on March 31, 2025, the FDA approved the freeze-dried formulation of JYNNEOS.
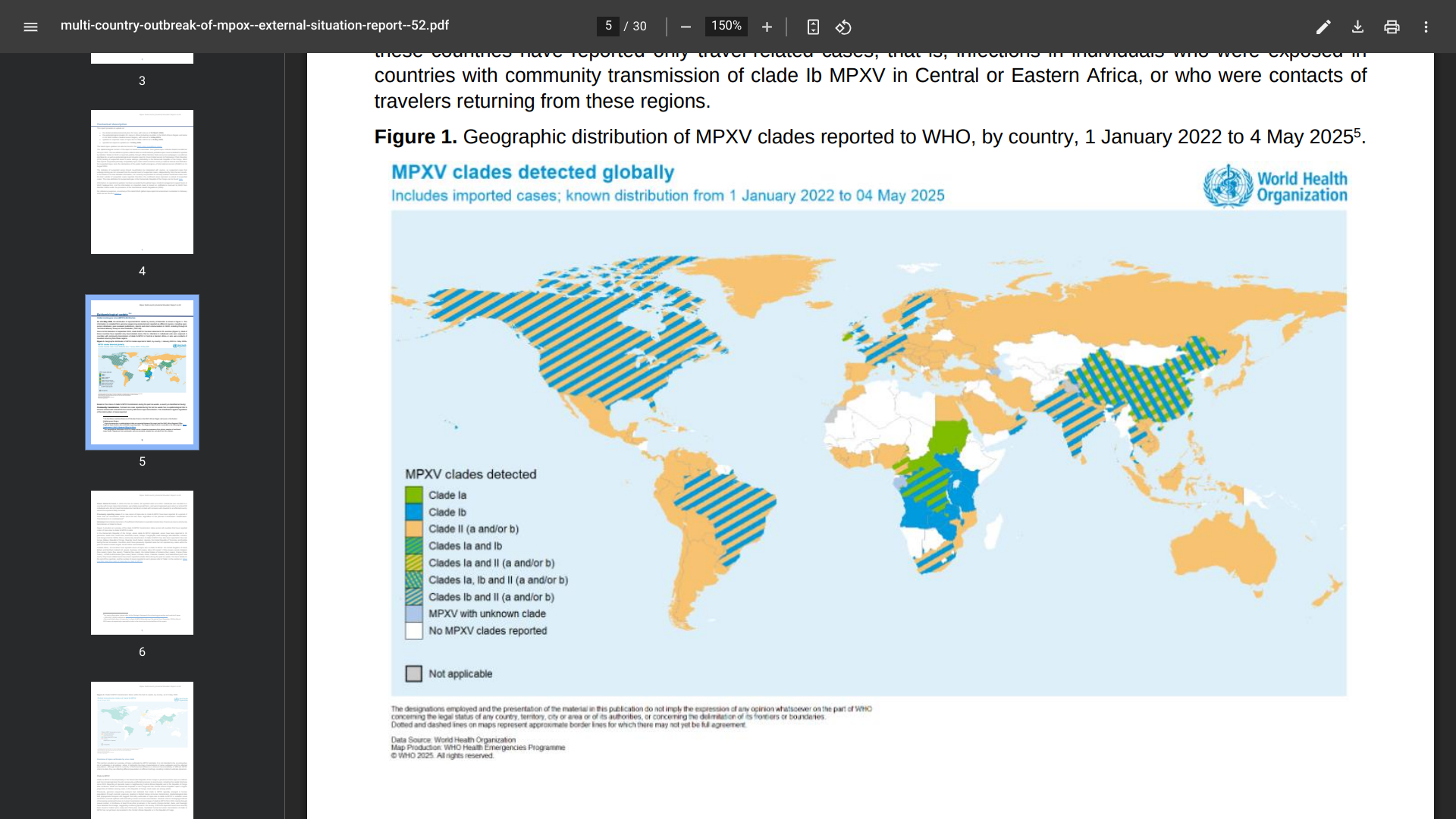
The latest surveillance data (Week #19) from Hong Kong showed that the overall local activity of COVID-19 has continued to increase, with some surveillance indicators having surpassed their highest levels in the past year.
In week 19, the weekly number of newly recorded positive nucleic acid test laboratory detections for the SARS-CoV-2 virus was 1,042, compared to 972 in the preceding week.
And COVID-19 outbreaks occurring in schools/institutions affected 52 people last week.
Furthermore, since January 2023 and May 10, 2025, the cumulative number of fatal cases with cause of death preliminarily assessed to be related to COVID-19 was 1,437.
As of May 14, 2025, the Centre for Health Protection (CHP) has been closely monitoring the local prevalence of SARS-CoV-2 variants in the neighboring regions of Hong Kong, including Singapore.
Sewage surveillance data showed an increasing trend in the prevalence of XDV in Hong Kong. XDV is a JN.1-related variant.
The latest information does not suggest that XDV will cause a more severe disease than JN.1, XBB, and their descendant lineages.
The public (and visitors) are advised to maintain strict personal and environmental hygiene to protect themselves against infection and prevent the spread of the disease.
Additionally, public members are advised to note the latest recommendations on using COVID-19 vaccines in Hong Kong. High-risk priority groups are recommended to receive a dose of a COVID-19 vaccine at least six months since the last dose or infection, regardless of the number of doses received previously. For more details, please visit this CHP link.
Hong Kong is a special administrative region of China on the southern coast. Recent data indicates over 40 million people visited Hong Kong last year.
If you depart from the United States, the CDC writes, ' Make sure you are up-to-date on all routine and travel vaccines, such as chikungunya and typhoid, before visiting Hong Kong in May 2025.'
With over 16 million visitors in 2024, the Republic of Singapore has become a tourist favorite destination. However, before visiting this city-state in Southeast Asia in May 2025, it is highly recommended that you protect yourself from COVID-19.
Like most other countries, Singapore has experienced multiple waves of COVID-19 since the first confirmed case in January 2020.
According to the Ministry of Health (MOH) and the Communicable Diseases Agency, COVID-19 infections have significantly increased in the Spring of 2025.
The MOH estimated the number of COVID-19 cases from the last week of April to May 3, 2025, at 14,200, compared to 11,100 cases in the previous week.
The 28% increase in cases could be due to several factors, including waning population immunity.
Currently, LF.7 and NB.1.8, descendants of the JN.1 variant, are the main COVID-19 variants circulating in Singapore, accounting for more than two-thirds of locally sequenced cases.
JN.1 is also the variant used in formulating the current COVID-19 vaccines, which remain effective in protecting against severe illness.
Singapore's MOH wrote that 'individuals at increased risk of severe COVID-19, such as those aged 60 years and above, medically vulnerable individuals, or residents of aged care facilities, are recommended to keep updated with vaccinations, and receive an additional dose around one year after their last dose.
In the United States, the U.S. FDA recently approved Nuvaxovid™, a non-mRNA, JN.1 COVID-19 vaccine that is active against current circulating strains, including KP.2 and KP.3. This vaccine, and other travel vaccines, are offered at clinics and pharmacies in the U.S.

The Government of Papua New Guinea recently reported two circulating vaccine-derived poliovirus type 2 (cVDPV2) cases in two healthy children from Lae, Morobe Province.
As of May 18, 2025, Papua New Guinea confirmed that cVDPV2 is a rare form of the virus that can emerge in under-immunised communities. The detection was made through routine environmental testing of wastewater collected from Bowerbird Road, China Town, Lae, in March 2025.
In a Facebook post, Health Minister Elias Kapavore described the situation as serious but manageable.
“We’ve dealt with this before and know what works,” Mr Kapavore said. “Vaccination is safe and effective, and we’re acting quickly to protect children.”
The planned response includes at least two rounds of nationwide vaccination. Parents and caregivers are strongly encouraged to bring their children for immunization during the upcoming campaigns.
If enough people in a community are immunized against polio, the virus will be deprived of susceptible hosts and will die out.
Furthermore, the Government stated it is 'committed to maintaining Papua New Guinea’s polio-free status.'
Last week, the Global Polio Eradication Initiative (GPEI) reported poliovirus cases and positive environmental isolates detected in Pakistan, Algeria, Burkina Faso, Chad, Côte d’Ivoire, Ethiopia, Somalia, and Sudan. As of May 14, 2025, more information on the countries and others is posted at this GPEI link.
The U.S. Food and Drug Administration (FDA) announced it has approved Novavax's Nuvaxovid COVID-19 vaccine.
As of May 17, 2025, Nuvaxovid is indicated in the U.S. to prevent COVID-19 in those aged 65 years and older and for those aged 12-64 years who have one or more underlying conditions that put them at a high risk of developing COVID-related severe outcomes.
On December 17, 2021, the World Health Organization granted an Emergency Use Listing for Novavax's vaccine, and it has been available under an emergency use authorization in the United States.
As of May 18, 2025, Novavax's vaccine is the only non-mRNA COVID-19 vaccine available at clinics and pharmacies in the U.S.

As the United States prepares for the next wave of COVID-19 disease, the government is taking steps to clarify what changes to the formula of preventive vaccines are needed.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) recently confirmed it will meet on May 22, 2025, in open session to discuss and recommend selecting the 2025-2026 formula for COVID-19 vaccines for use in the U.S.
This VRBPAC meeting will be held virtually, from 8:30 a.m. to 4:30 p.m. ET, and is open to the public to attend digitally.
The U.S. Food and Drug Administration (FDA) intends to make background material available to the public no later than two business days before this meeting.
Recently, the new FDA Commissioner Marty Makary stated that Vinay Prasad, the director overseeing vaccines, intends to clarify the FDA’s expectations for vaccine development and approval.
For example, the FDA has asked Novavax Inc. to conduct a new randomized controlled trial for its non-mRNA COVID-19 vaccine.
Earlier this week, the World Health Organization (WHO) Technical Advisory Group on COVID-19 Vaccine Composition advised manufacturers that monovalent JN.1 or KP.2 vaccines remain appropriate vaccine antigens; monovalent LP.8.1 is a suitable alternative vaccine antigen.
The WHO wrote, 'Overall, ' the currently approved monovalent JN.1 or KP.2 vaccines continue to elicit broadly cross-reactive immune responses to circulating JN.1-derived variants.'
As of May 16, 2025, COVID-19 vaccines are available at various clinics and pharmacies in the U.S.

With the various countries reporting chikungunya virus outbreaks in 2025, the U.S. government has announced reassuring news regarding disease prevention.
In April, the independent vaccine committee issued recommendations for using chikungunya vaccines. Effective May 13, 2025, the Health and Human Services (HHS) Secretary adopted the recommendations, which are now the official recommendations of the U.S. CDC.
The Advisory Committee on Immunization Practices (ACIP) recommends the live attenuated chikungunya vaccine for persons aged ≥18 years traveling to a country or territory with a chikungunya outbreak. In addition, the live attenuated chikungunya vaccine may be considered for persons aged ≥18 years traveling or residing in a country or territory without an outbreak but with elevated risk for U.S. travelers if planning travel for an extended time, such as 6 months or more.
Additionally, the ACIP recommends the virus-like particle chikungunya vaccine for persons aged ≥12 years traveling to a country or territory where there is a chikungunya outbreak. In addition, the virus-like particle chikungunya vaccine may be considered for persons aged ≥12 years traveling or taking up residence in a country or territory without an outbreak but with elevated risk for U.S. travelers if planning travel for an extended period, such as six months or more.
As of May 15, 2025, travel vaccination appointments are commercially available at clinics and pharmacies in the U.S.

Led by a team from the U.S. Centers for Disease Control and Prevention (CDC), the findings from a study published in the Journal of Infectious Diseases demonstrated the feasibility of assessing seasonal influenza vaccine effectiveness (VE) using linked immunization and laboratory data from public health surveillance systems.
Published on May 13, 2025, among 1,382,142 laboratory reports, 129,253 persons (9%) (129,253) had a positive influenza test result, of whom 415,390 (30%) had documented influenza vaccination ≥14 days before test date. VE against laboratory-confirmed influenza was 41% (95% confidence interval (CI), 40%–42%). VE was 32% (95% CI, 31%-33%) against influenza A, 68% (95% CI, 66%-69%) against influenza B.
Among older adults aged 65 years or more, flu shot VE was 26% (95% CI, 24%–29 %).
The authors wrote, "Differences in viral evolution may contribute to waning vaccine effectiveness or immune escape."
"Age-related factors contributing to lower VE might include diminished adaptive immune response, increased prevalence of comorbidities, and frailty."
They also noted that the flu shot's lower VE estimates with increasing patient age are consistent with a meta-analysis of test-negative studies conducted from 2004 to 2015.
As of May 9, 2025, the U.S. CDC confirmed that seasonal influenza activity for 2024-2025 is declining. And the CDC continues to recommend that everyone ages 6 months and older get an annual flu vaccine as long as influenza viruses are circulating.