Search API
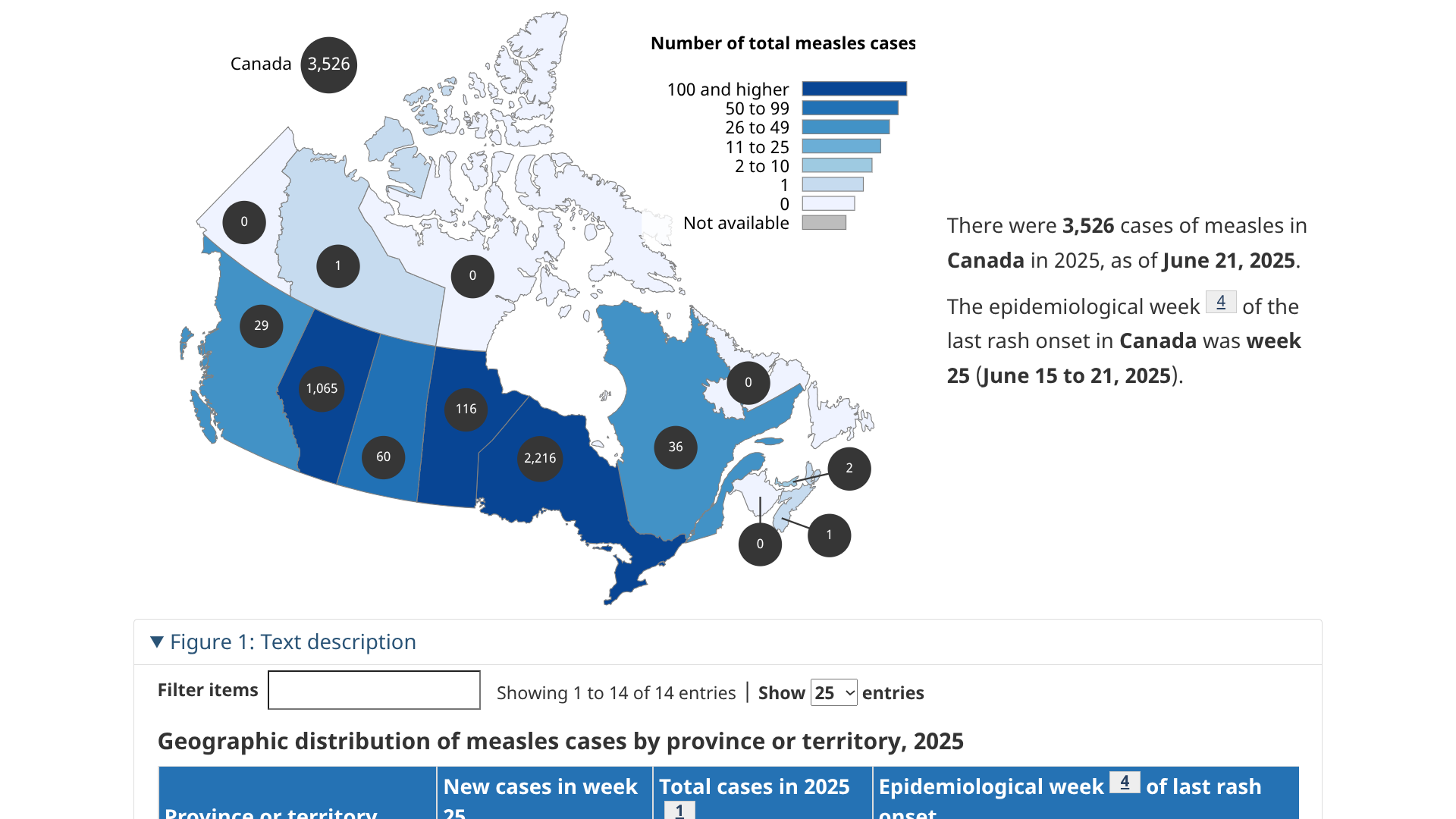
A multijurisdictional measles outbreak has occurred in Canada, beginning in New Brunswick in October 2024. This year, it has spread in Ontario (1,949), and related cases have been reported in Alberta, British Columbia, Manitoba, the Northwest Territories, Nova Scotia, Prince Edward Island, Quebec, and Saskatchewan.
Canada Health's Measles and Rubella Weekly Monitoring Report states that, of the 2,755 measles cases (2,429 confirmed, 326 probable) reported in 2025, 1,867 cases are linked to this outbreak as of June 2, 2025.
At the end of May 2025, only 244 new measles cases were reported in one week, indicating a continued decline of this outbreak.
The government says, 'If you're infected with the measles virus, you can spread it to others. This is possible from 4 days before the onset of the rash to 4 days after the rash begins. Isolate at home and call a health care provider immediately. They will advise you on what to do.'
As of June 8, 2025, measles vaccination services are offered throughout Canada.
While about half of the world’s population is at risk of malaria, the African Region accounts for about 95% of all cases.
However, this mosquito-transmitted disease has been detected in various unusual locations in 2025.
In the Western Pacific region, which includes Australia, Papua New Guinea (PNG), New Zealand, Vanuatu, and the Solomon Islands, malaria cases have been reported.
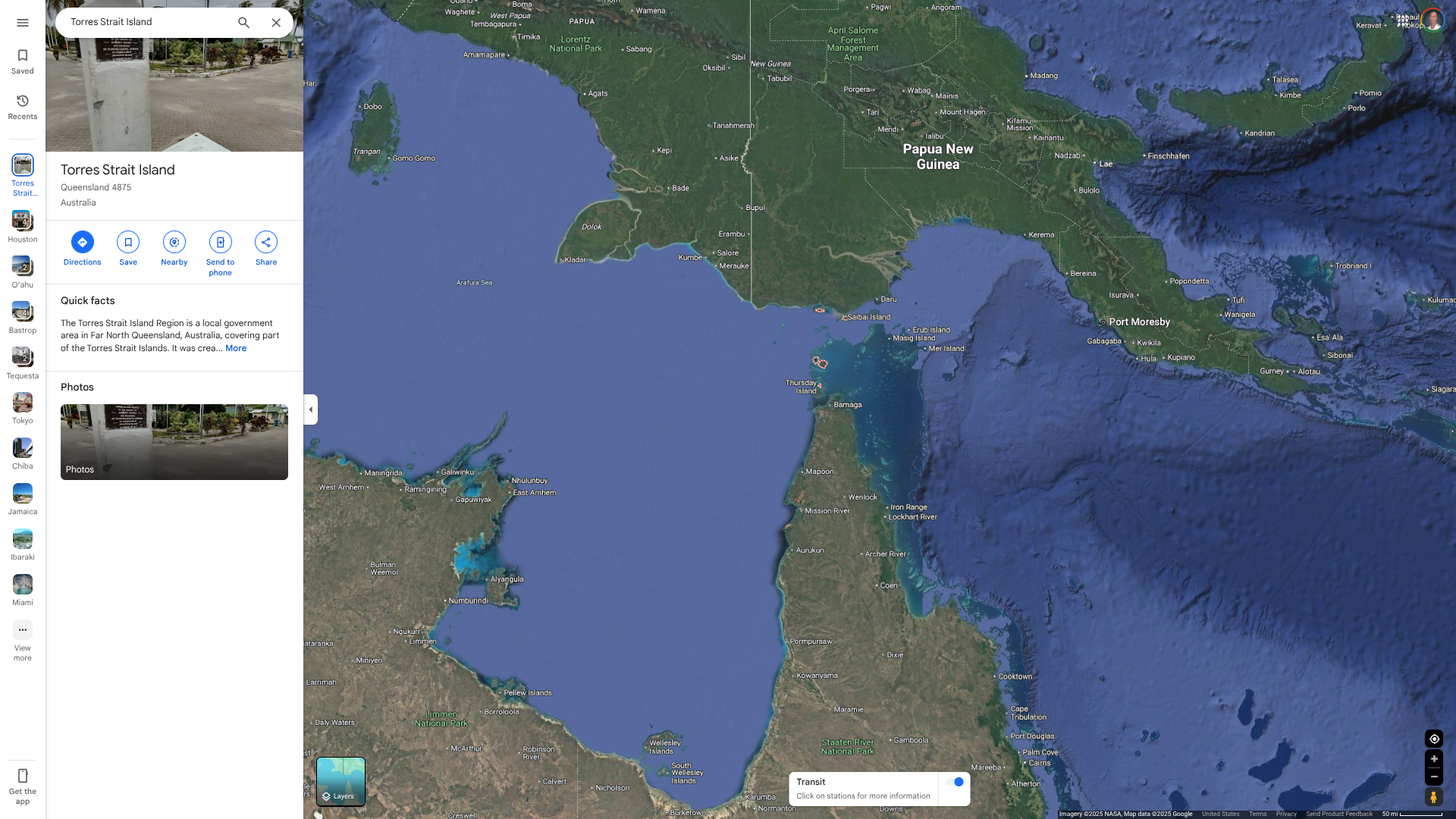
Malaria in Australia is commonly recorded in returned international travellers, with about 100 imported cases recorded in Queensland each year. But so have locally acquired cases.
For example, Queensland Health recently reported a second locally acquired malaria case in a Torres Strait LGA resident.
As of June 2, 2025, investigations into this malaria case are ongoing.
In 2025, 71 malaria notifications were reported, of which 97% were related to international travel, predominantly with PNG and the Solomon Islands.
The last locally acquired malaria outbreak in the Torres Strait was in 2023, with five cases detected in northern Torres Strait Islands.
From a disease prevention perspective, neither malaria vaccine is offered in Australia as of June 6, 2025.
According to a Case Report published by the Journal of Infection and Chemotherapy (Volume 31) in June 2025, Japanese encephalitis (JE) has emerged in a previously non-reported area in Japan, suggesting that the number of JE patients may be underestimated in Japan.
This report describes three cases of JE in a single hospital in Narita over three years.
These researchers suggest that physicians in Japan should consider JE as a differential diagnosis in 2025 when encountering cases of encephalitis or meningitis with unknown etiology during the warm season, even in areas where JE has not been previously reported.
This prefecture is located a few miles east of Tokyo, a city with over 35 million residents.
JE is an infection of the brain caused by the Japanese encephalitis virus, which is spread to people through the bite of an infected mosquito or close contact with livestock, such as birds, goats, and pigs.
In Japan, JE used to be endemic, and more than 1000 JE patients had been reported before the 1960s.
However, the introduction of the JE vaccine in 1954 and its widespread use in childhood vaccination from 1967 dramatically reduced the disease burden. As of 2022, only one case had been reported in Chiba and Ibaraki prefectures, respectively, in the past 10 years, and both cases occurred in areas famous for pig farming, far from Narita City.
As of June 5, 2025, the U.S. CDC recommends JE vaccination for specific people visiting Japan.
The CDC recommends vaccination for travelers who are moving to an area with JE, spend long periods in areas with JE present, or frequently travel to those areas in Japan.
JE vaccination is not recommended for travelers planning short-term trips to urban areas or those traveling to places with no clear JE season.
When departing for Japan or any other JE outbreak area, such as Australia, Valneva SE's IXIARO® (JESPECT®) vaccine is commercially offered at travel clinics and pharmacies in 2025.
With a population of 2.8 million, Jamaica remains a popular tourist destination in June 2025. Last year, this Caribbean Island destination welcomed over 4 million visitors by air and sea.
A short two-hour flight from Miami, Florida, brings tourists to vast resorts and warm waters.
While the U.S. Department of State recently reduced its Level 3 travel advisory for Jamaica, it still advises Americans to exercise caution while visiting in June 2025.
As of May 29, 2025, the State Department's periodic review indicates that civil unrest in Jamaica has decreased since 2024; however, it remains statistically high throughout the country.
Tourist areas typically experience lower rates of crime compared to other parts of the country. Still, the homicide rate reported by the Government of Jamaica is among the highest in the Western Hemisphere.
From a local health perspective, U.S. citizens should not expect the same level of healthcare services in Jamaica. This concern includes slower emergency service response times and reduced availability of care for illnesses or injuries.
Private hospitals typically require payment upfront before admitting patients and may not have the necessary resources to provide specialized care.
The U.S. Embassy in Jamaica previously stated 'Be aware that U.S. Medicare/Medicaid does not apply overseas. Most hospitals and doctors overseas do not accept U.S. health insurance. U.S. citizens with medical emergencies can face bills in the tens of thousands of dollars, with air ambulance service to the United States.'
We highly recommend that you purchase insurance before traveling, writes the Embassy, which is located at 142 Old Hope Road, Kingston 6.
Seperately, the U.S. CDC advises visitors to take actions to protect themselves from diseases such as measles and chikungunya.
Furthermore, the Pan American Health Organization has been working since 2003 to control and prevent dengue. The disease remains a substantial concern throughout the Americas, but only 165 cases have been reported in Jamaica this year.
The CDC recommends checking the vaccines and medicines list and visiting your doctor at least a month before your trip to Jamaica.
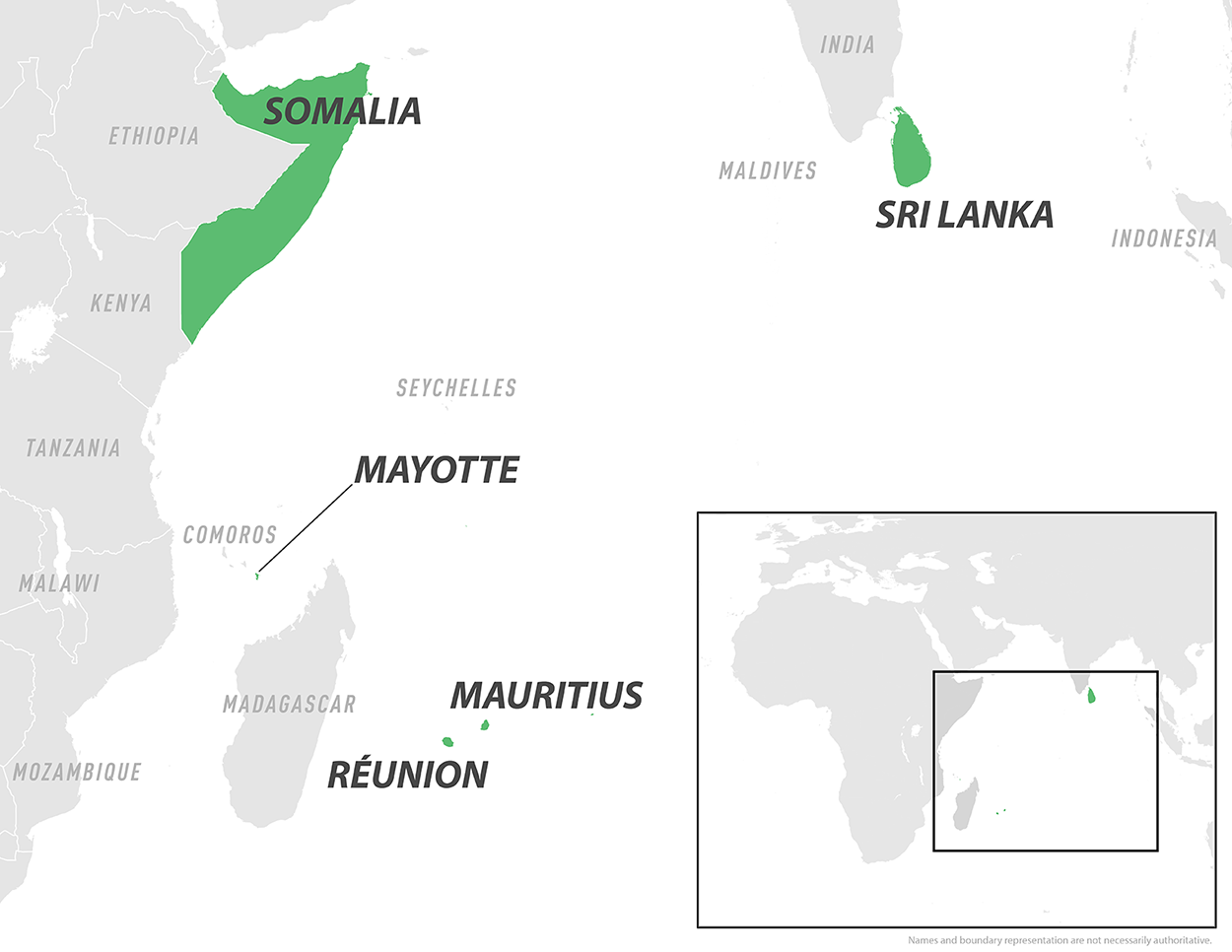
Since late May 2025, France's overseas department of Mayotte has experienced a significant outbreak of the mosquito-transmitted Chikungunya virus throughout the island.
As of June 2, 2025, Public Health France reports a total of 560 confirmed cases of chikungunya recorded during this phase of the ORSEC arbovirus plan.
From a location perspective, there has been a persistent concentration of cases in the municipalities of Mamoudzou, Pamandzi, and Dzaoudzi.
These are Mayotte's first locally transmitted cases of chikungunya since the 2005–2006 outbreak, which resulted in approximately 7,300 cases.
According to the World Health Organization (WHO), Chikungunya outbreaks have been documented on islands within and countries bordering the Indian and Pacific Oceans, such as Mauritius, Réunion, Somalia, and Sri Lanka, in 2025.
Public health response measures have included targeted vaccination efforts with Valneva SE's IXCHIQ® vaccine, the first approved monovalent, single-dose, live-attenuated vaccine.
While the WHO states that no measures related to international traffic and trade are warranted at this time, the U.S. CDC has updated its information to a Level 2 - Practice Enhanced Precautions, Travel Health Advisory.
The CDC recommends vaccination for travelers visiting an area with a chikungunya outbreak. Furthermore, if you are pregnant, consider reconsidering travel to the affected areas, particularly if you are nearing the delivery of your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
In addition to chikungunya, another mosquito-transmitted disease has been spreading on Mayotte. The circulation of dengue fever on the island has reached 21 confirmed cases since the beginning of 2025.
Before visiting Mayotte in June 2025, the CDC recommends consulting with a travel vaccine specialist to discuss immunization options.
ImmunityBio, Inc. recently announced that the U.S. Food and Drug Administration (FDA) has granted Expanded Access authorization for the use of its Cancer BioShield™ platform, anchored by ANKTIVA®, to treat lymphopenia in adult patients with refractory or relapsed solid tumors independent of tumor type who have progressed after first-line standard-of-care treatment, chemotherapy, radiation, or immunotherapy.
On June 2, 2025, ImmunityBio stated 'while oncologists and patients have long had therapies such as EPOGEN® and NEUPOGEN® to manage chemotherapy- and radiation-induced anemia and neutropenia, no comparable option has been available for lymphopenia.'
To date no treatment exists for lymphopenia, a depletion of critical lymphocytes responsible for immunogenic cell death, specifically natural killer (NK) cells, killer CD8+ T cells and CD4+ with memory T cells.
Treatment-induced lymphopenia is a debilitating consequence of chemotherapy, radiation, specific immunotherapies, and steroids. This treatment-acquired immunodeficiency not only increases susceptibility to infections but also deprives the body’s immune system to fight residual or recurrent cancer, accelerating metastasis and disease progression, and contributing to early mortality.
Countless publications over the last two decades have reported lymphopenia as a highly predictive biomarker of poor prognosis across all tumor types.1-6 Despite its significant clinical impact, the pharmaceutical industry has largely overlooked lymphopenia as a disease in its own right, and no approved therapies have existed to directly address it, until the approval of ANKTIVA in the treatment of BCG-unresponsive bladder cancer with the mechanism of action of an IL-15 superagonist proliferating key lymphocytes.
Dr. Patrick Soon-Shiong, Founder, Executive Chairman, and Global Chief Scientific and Medical Officer of ImmunityBio, commented in a related press release, “This FDA authorization allows all patients with solid tumors suffering from immune collapse following first-line therapy of chemo, radiation, or immunotherapy to access ANKTIVA."
"The survival benefit we observed at ASCO 2025 in 3rd to 6th line advanced metastatic pancreatic cancer confirms that restoring lymphocyte levels—rather than depleting them—can change the course of disease.”
As of June 4, 2025, ANKTIVA is available at participating cancer centers in the United States and other countries, such as the Kingdom of Saudi Arabia.

With measles cases being reported in many countries this year, the U.S. Centers for Disease Control and Prevention (CDC) issued a Travel Health Advisory highlighting updated vaccination guidance regarding this highly contagious respiratory illness.
As of May 28, 2025, the CDC states that international travelers are at risk of contracting measles if they have not been fully vaccinated at least two weeks before departure or have not had measles in the past.
Therefore, the CDC recommends that 'all international travelers should be fully vaccinated against measles with the measles-mumps-rubella (MMR) vaccine, according to the CDC's measles vaccination recommendations for international travel.
This is essential guidance since most people who bring measles into the United States are unvaccinated U.S. residents who get infected during international travel.
Currently, significant measles outbreaks have been confirmed in the Region of the Americas, including Canada (2,755 cases), Mexico, and Texas.
While the Texas measles outbreak has significantly slowed, the outbreak in Ottawa, Canada, continues to expand, with 1,949 cases reported.
As of June 4, 2025, various measles-containing vaccines are available at health clinics and pharmacies in the United States.
The Republic of Ecuador has been exposed to a wide range of zoonotic pathogens that have a significant impact on the population's health and the overall economy.
Recent disease outbreaks, such as yellow fever, rabies, hantavirus, and highly pathogenic avian influenza A(H5N1), have disrupted the health system of this South American country.
For example, the number of yellow fever cases reported in South America so far in 2025 represents a threefold increase compared to the cases reported in 2024.
Since the beginning of 2025 and as of early May, four confirmed fatal cases of yellow fever have been reported in Ecuador, from the provinces of Morona Santiago (one fatal case) and Zamora Chinchipe (three fatal cases).
These conditions led Ecuador to establish priority surveillance, prevention, and control strategies for potential outbreaks of animal-borne diseases.
In response to these conditions, with technical support from the Pan American Health Organization/World Health Organization, specialists from the Ecuador Ministry of Public Health, and others announced on June 2, 2025, they are working to prioritize zoonotic and emerging diseases to obtain a list that would provide the country with clarity in the implementation of surveillance and control tasks for these pathologies.
To alert international visitors, the U.S. CDC has included Ecuador in various disease outbreak alerts.
The CDC recommends yellow fever vaccination for travelers aged 9 months or older traveling to areas below 7,550 feet in elevation, east of the Andes Mountains, in the provinces of Morona-Santiago, Napo, Orellana, Pastaza, Sucumbíos, Tungurahua, and Zamora-Chinchipe.
As of June 3, 2025, the YF-Vax vaccine is generally not recommended for travel to areas with an elevation below 7,550 ft.
The vaccine is required for most travelers arriving from Brazil, the Democratic Republic of the Congo, or Uganda, including those with 12-hour airport transits or layovers in any of these countries. But not those arriving from the U.S.
Located on South America's Pacific Ocean, Ecuador also includes the Galápagos Province, about 600 miles west of the mainland.
This travel vaccine, along with others, is commercially offered at clinics and pharmacies in the U.S.