MTBVAC Tuberculosis Vaccine
MTBVAC Tuberculosis Vaccine Clinical Trials, Dosage, Indication, Side Effects
Biofabri, S.L. MTBVAC (BBV169) is a live-attenuated M. tuberculosis (TB) vaccine candidate that has been evaluated in Phase 3 clinical trials. MTBVAC is the first vaccine against TB derived from a human source and has demonstrated safety, efficacy, and immunogenicity in preclinical and clinical studies. MTBVAC was designed by the research group of Dr. Carlos Martín Montañés from the Department of Microbiology at the University of Zaragoza, Spain, and Brigitte Gicquel from the Institut Pasteur, Paris. This vaccine is designed to provide improved efficacy compared to the 100-year-old Bacille Calmette-Guérin (BCG) vaccine, which is used for TB treatment. MTBVAC is the only vaccine against TB in clinical trials based on a genetically modified form of the pathogen, Mycobacterium tuberculosis, isolated from humans. Unlike BCG, it contains all the antigens present in strains that infect humans.
MTBVAC is based on a rationally attenuated M. tuberculosis clinical isolate belonging to modern lineage four that conserves most of the T cell epitopes described for TB, including the antigens ESAT6 and CFP10 of the RD1, while incorporating two independent stable genetic deletions of the phoP and fadD26 genes. MTBVAC stimulates specific immune responses that mimic natural tuberculosis infection without causing disease. A uniquely attenuated M. tuberculosis strain was generated by genetic engineering, with an antigen repertoire similar to that of virulent M. tuberculosis and containing known human T cell epitopes present in M. tuberculosis but absent in BCG.
In a phase 1/2 study, MTBVAC had acceptable reactogenicity and induced a durable CD4 cell response in infants. MTBVAC completion of Phase 2 dose-finding trial and a double-masked, controlled Phase 3 clinical trial in newborns started in 2023, comparing the vaccine with the current BCG vaccine. Bharat Biotech International Limited, in collaboration with Biofabri, has begun a series of clinical trials to evaluate the safety, immunogenicity, and efficacy of MTBVAC in India.
On February 26, 2025, IAVI and Biofabri announced that the first doses of MTBVAC were administered in the IMAGINE (Investigation of MTBVAC toward Accelerating Global Immunization for a Neglected Epidemic) Phase 2 clinical trial. The trial's first participants were vaccinated at Be Part Research Limited in Paarl, South Africa. The IMAGINE trial is a large-scale safety and efficacy clinical trial of the TB vaccine candidate MTBVAC. The study is funded by Open Philanthropy, the Bill & Melinda Gates Foundation, and the German Federal Ministry of Education and Research through the KfW Development Bank. Results are expected to be published in 2025. If MTBVAC is shown to be efficacious, Biofabri, IAVI, and other partners will collaborate to ensure a sufficient and affordable supply of MTBVAC is available for low- and middle-income countries.
Biofabri was founded in 2008 and is part of Zendal, a Spain-based biopharmaceutical group comprising seven companies focused on researching, developing, manufacturing, and marketing high-value-added products in the healthcare industry. MTBVAC was designed by the Spanish researcher Carlos Martin from the University of Zaragoza and Brigitte Gicquel, Ph.D., of Institut Pasteur.
MTBVAC Vaccine Indication
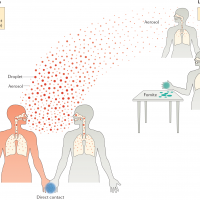
MTBVAC is being developed for two purposes: as a more effective, potentially longer-lasting vaccine than BCG in newborns, and to prevent TB disease in adults and adolescents, for whom there is currently no effective vaccine. Tuberculosis is an infectious disease caused by the bacterium Mycobacterium tuberculosis (M. tuberculosis). M. tuberculosis in inhaled droplets enters the alveolar passages of exposed humans, contacting resident cells, and is ingested by the alveolar macrophages.
MTBVAC Vaccine News
February 26, 2025 - Lewis Schrager, M.D., head of IAVI's TB vaccine development efforts, stated in a press release, "Because MTBVAC is a single-shot vaccine, we are hopeful that, if proven effective, MTBVAC could prevent millions of cases of TB disease, particularly in persons living in some of the world's most difficult-to-reach locations."
March 23, 2024 - Bharat Biotech International Limited, in collaboration with Biofabri, has started a series of clinical trials to evaluate the safety, immunogenicity, and efficacy of MTBVAC in India.
December 18, 2023 - IAVI and Biofabri announced that the Bill & Melinda Gates Foundation has awarded IAVI US$55 million to conduct a Phase IIb trial assessing the safety and efficacy of the MTBVAC vaccine.
January 4, 2021 - Article - MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies.
January 2016 - The MTBVAC vaccine is safe and immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice.
MTBVAC Vaccine Clinical Trials
Two Phase 2 trials of MTBVAC have been completed, one sponsored by Biofabri and supported by the European and Developing Countries Clinical Trials Partnership in infants in South Africa, and one hosted by IAVI and supported by the U.S. National Institutes of Health and the U.S. Department of Defense through its Congressionally Directed Medical Research Program.
In India, Phase 2 Clinical Trial Protocol [BIO/CT/24/000042] for the Mycobacterium Tuberculosis (Live Attenuated) Vaccine. As of March 30, 2024, the revised Phase II protocol should be submitted to CDSCO for approval.
A phase 3 trial (NCT04975178) is underway to evaluate the efficacy, safety, and immunogenicity of MTBVAC compared to BCG in healthy, HIV-unexposed, and HIV-exposed uninfected newborns in tuberculosis-endemic regions of sub-Saharan Africa. This randomized, double-blind, controlled trial aims to enroll 7120 newborns who have not received BCG vaccination and have not been exposed to Mycobacterium tuberculosis. The trial is being conducted at six sites, including four in South Africa, one in Madagascar, and one in Senegal.
A Phase 2 study in HIV-infected adults started in 2024 to determine whether MTBVAC is safe in this population. This ongoing trial at 16 sites in South Africa – involving the vaccination of 276 adults – is evaluating safety and immunogenicity in HIV-negative and HIV-positive adults and adolescents vaccinated with MTBVAC.
Phase 2b/3 trials in adolescents and adults are planned for 2025 in various regions worldwide.