Search API
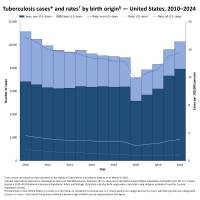
Valneva SE today reaffirmed that the Phase 3 clinical trial of its Lyme disease vaccine candidate, VLA15, remains on track.
The company's press release on October 6, 2025, states that Participants in the VALOR clinical trial will be monitored for the occurrence of Lyme disease cases until the end of 2025. Valneva expects the VALOR trial outcomes to be announced in the first half of 2026, followed by planned regulatory submissions.
Valneva's development partner, Pfizer Inc., continues to aim to submit a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) and a Marketing Authorization Application to the European Medicines Agency in 2026, pending the receipt of positive Phase 3 data.
Pending approval, Valneva expects Pfizer to launch the vaccine in the second half of 2027.
The FDA granted the VLA15 vaccine development program Fast Track designation in July 2017, and it remains the leading candidate in development.
The VLA15 vaccine protects humans by raising antibodies that prevent Borrelia from migrating from ticks after a bite. VLA15 is designed to cover about 97% of Borrelia in North America and Europe. VLA15 is being tested as an alum-adjuvanted formulation and administered intramuscularly.
While Lyme disease has been found in the northeastern USA for decades, ticks in the upper Midwest are now spreading this severe disease.
Residents of Prince Edward Island, Canada, were recently informed that a small tapeworm, primarily found in foxes and coyotes, known as Echinococcus multilocularis, has been detected at high levels.
Research conducted by the Atlantic Veterinary College shows that between 20 to 30% of red foxes and coyotes on the Island carry the parasite. Although rare, Echinococcus multilocularis can cause a disease in humans called alveolar echinococcosis.
The parasite is mainly spread through the feces of infected animals such as foxes, coyotes, and occasionally dogs.
Humans can become infected if they accidentally ingest microscopic eggs in contaminated food, water, soil, or through close contact with animals that have been exposed to the parasite.
"While it's essential to be aware, this is not a cause for alarm. Even in parts of Canada and Europe where this parasite has been present for some time, human infections are rare. Preventive steps, such as washing hands, washing or cooking wild-picked foods, and consulting your veterinarian about deworming your pets, are highly effective, according to a statement by Dr. Marguerite Cameron, an epidemiologist with the Chief Public Health Office.
As of September 15, 2025, Canadian public health officials emphasize that the risk to human health remains extremely low, even in regions where the parasite is well-established. Additional details were disclosed on October 3, 2025.
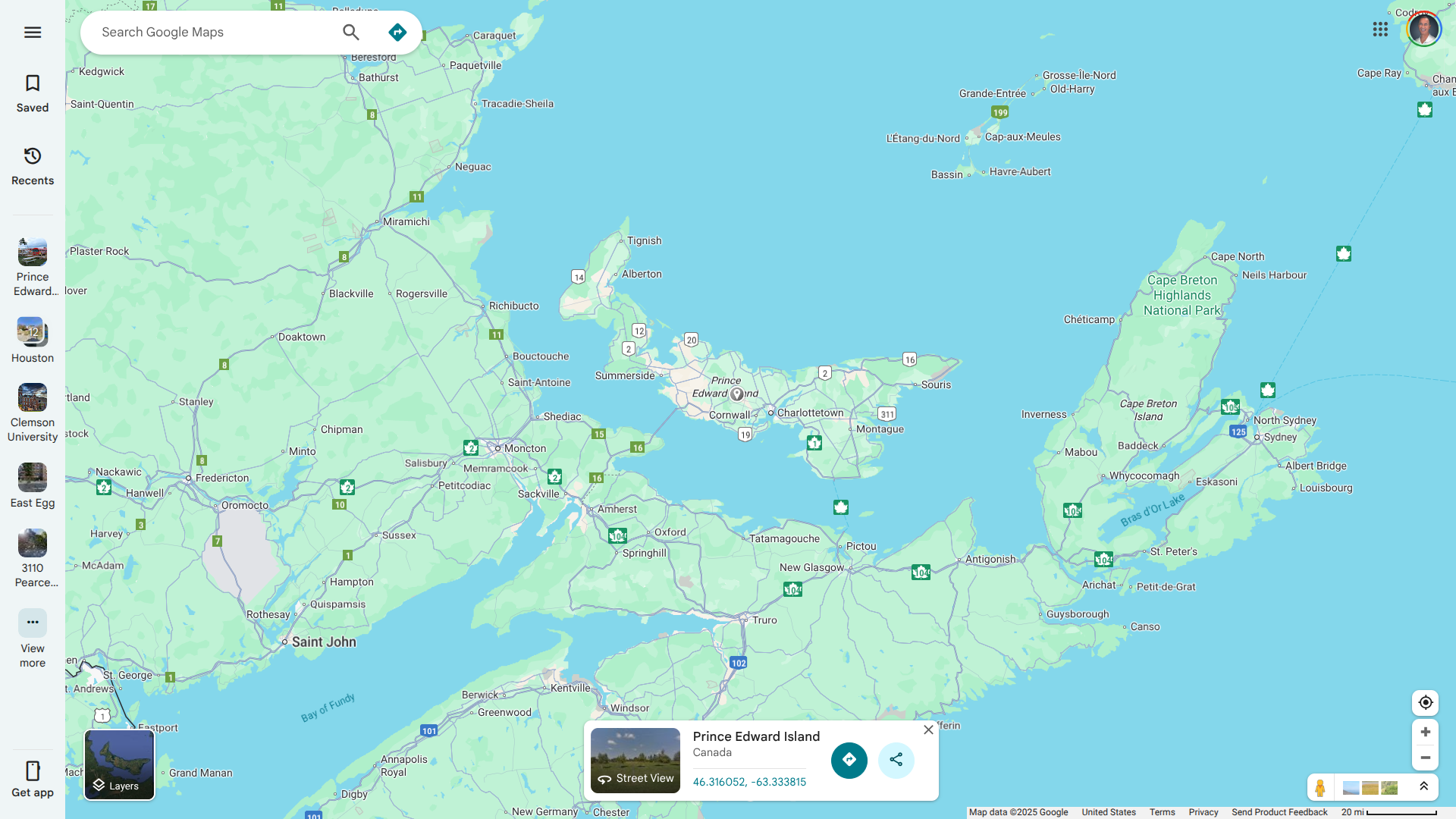
The South Carolina Department of Public Health (DPH) recently confirmed an outbreak of measles in the Upstate area.
As of October 1, 2025, a total of eight measles cases have been reported. Five out of the eight instances became sick within the past month.
Currently, cases are following DPH isolation guidance to prevent further spread of the vaccine-preventable virus near Clemson University, which has a student population exceeding 7,000.
"Measles is highly contagious, and there is risk for continued, rapid spread of the disease in the Upstate among communities with low immunization rates," said Dr. Linda Bell, state epidemiologist and Health Programs Branch director, in a press release.
"Measles-mumps-rubella (MMR) vaccination remains the most important tool for preventing measles infection and spread. We strongly encourage everyone to review their immunization records and make sure they are up to date on all recommended vaccinations, including the MMR."
"The unknown source of two of the cases indicates unrecognized community spread," said Dr. Bell. "We anticipate more cases will be identified and implore community members to act responsibly. If you are ill, stay home."
South Carolina is not alone in reporting measles outbreaks.
As of September 2025, over 1,500 confirmed measles cases have been reported across 41 states, according to the Centers for Disease Control and Prevention data. The majority of measles cases were reported in Texas.
Additionally, both Canada and Mexico continue to report measles outbreaks in 2025.
According to the World Health Organization (WHO), India is the tuberculosis (TB) capital of the world, reporting about 2.5 million new cases annually. TB is the country's most fatal infectious disease, with an estimated 500,000 related fatalities every year.
The WHO says eliminating TB depends on early, accurate, and universal detection to reduce community transmission of this airborne disease.
To help reduce this significant health issue, the Indian Council of Medical Research has recently validated innovative tools from Huwel Lifesciences: the Quantiplus MTB FAST detection kit and the UniAMP MTB Nucleic Acid Test Card.
A recent study concluded that the Quantiplus assay demonstrated sensitivity and specificity of 86% and 96%, respectively, for the detection of pulmonary Mycobacterium tuberculosis (M. tuberculosis) in sputum samples, compared to liquid culture, and showed significant improvement with the Xpert MTB/RIF assay.
The diagnostic performance of the Quantiplus® assay is comparable to that of the Truenat MTB assay reported earlier. The limitation of the assay is that it requires a clean environment to avoid cross-contamination.
These new technologies promise to transform TB detection, making it faster, more affordable, and broadly accessible across India.
The 100-year-old Bacillus Calmette-Guérin (BCG) vaccine is the primary TB vaccine used in India. BCG provides partial protection against TB infection, especially in high-risk populations. It is administered to infants as part of the National Immunization Program.
India is actively involved in the development of new TB vaccines, such as MTBVAC and VPM1002. These vaccine candidates aim to provide broader and more durable protection against TB.
In the United States, access to the FDA-approved BCG vaccine is limited even as TB cases continue to increase.

While the United States has witnessed a reduction in Mpox cases in 2025, the city of Chicago's recent spike in cases has not abated.
According to the Chicago Department of Public Health (CDPH), from June to September 30, 2025, 104 cases of Mpox have been reported. Those are more cases than were reported over the same time period in 2023 (40) and 2024 (53) combined.
The median age of those diagnosed with the sexually transmitted Mpox virus over the past four months is 34, of whom most are men.
The CDPH data underscore the fact that this is a critical time for the mpox vaccination effort to limit new Mpox cases in Chicago, IL.
As of October 4, 2025, the U.S. Centers for Disease Control and Prevention (CDC) has not issued a Travel Health Notice regarding this Mpox outbreak.
CDPH is co-sponsoring several mpox vaccination events throughout October 2025.
Vaccination is especially encouraged for sexually active gay and bisexual men, who are most at risk.
The CDC-recommended Mpox vaccine (JYNNEOS®) is available at CDPH Sexual Health Clinics and throughout the USA.
Globally, the World Health Organization (WHO) recently published its 58th situation report on the multi-country outbreak of mpox. As of September 19, 2025, the WHO reported that 59 countries confirmed a total of 3,780 cases, including 15 related fatalities. Both clades of the monkeypox virus continue to circulate in various countries.

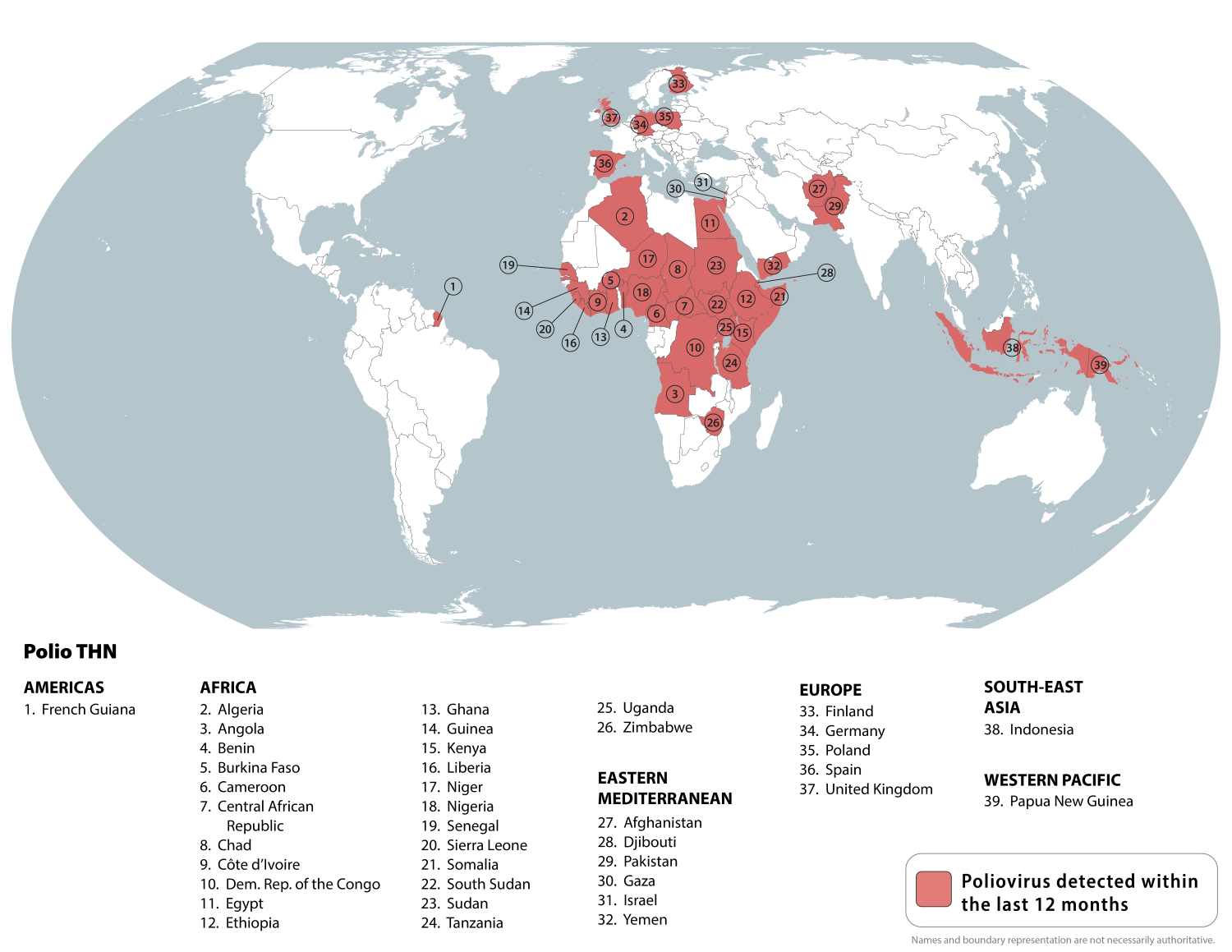
The World Health Organization's Strategic Advisory Group of Experts on Immunization (SAGE) recently endorsed two critical innovations for polio eradication. SAGE strongly emphasized that polio eradication cannot be achieved solely through technical interventions.
As of the end of September 2025, the SAGE recommended that fractional doses of Sabin-based inactivated polio vaccine (IPV) be used in the same way as fractional doses of Salk-based IPV.
This recommendation should stretch the vaccine supply and reach more children.
SAGE also supported the broader rollout of novel oral polio vaccine type 2 (nOPV2) to help prevent persistent outbreaks of circulating variant poliovirus type 2 (cVDPV2) in some of the most challenging areas.
SAGE stated it is very concerned about the continued transmission of wild poliovirus (WPV1) in Pakistan and Afghanistan.
Last week, Pakistan reported three cases of WPV1.
While improving routine immunization coverage, vaccination campaigns, surveillance, and outbreak response remain essential, the decisive factor is sustained national political leadership and accountability at every level, added SAGE.
In the United States, the IPV remains the only polio vaccine offered at clinics and pharmacies.
The U.S. CDC states that before traveling to any at-risk destination, adults who have previously completed the whole, routine polio vaccine series may receive a single, lifetime booster dose of the IPV polio vaccine. Polio vaccination services are offered at clinics and pharmacies in the USA.
In Italy, where the West Nile virus (WNV) has become endemic, a total of 718 confirmed cases, including 49 related fatalities, have been reported in 2025.
As of October 3, 2025, the most affected regions are Lazio (252 cases), Campania (124 cases), and Veneto (91 cases).
As of September 2025, Italy accounted for 76.3% of all reported human cases and for 79.6% of all reported outbreaks in equids and birds, underscoring the significant WNV activity in the country.
Italy is not alone in reporting mosquito-transmitted WNV cases this year in Europe, as Albania, Bulgaria, France, Greece, Hungary, Romania, Serbia, Spain, and Türkiye have confirmed cases to the European Centre for Disease Prevention and Control.
Following several years of research, WNV vaccines remain unavailable in 2025.