Search API
RedHill Biopharma today announced that it had received a contract with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to advance the development of opaganib, a small-molecule treatment for Ebolavirus.
This novel, potentially broad-acting drug has shown mutation-resistant antiviral and anti-inflammatory activity, likely to counteract the vascular impacts of Ebola infection.
In a press release on October 14, 2024, the company stated that it is pursuing an animal-rule pathway for potential approval for this Ebola treatment candidate. This process is used when human clinical trials are not ethical or feasible.
Guy Goldberg, RedHill's Chief Business Officer, commented, "Currently, only Inmazeb™, a combination of three monoclonal antibodies, and Ebanga™, a single monoclonal antibody, are FDA-approved to treat Ebola infections. As such, there is an urgent need for additional effective and easy-to-distribute and administer therapies (during an outbreak)."
While there are approved Zaire Ebola vaccines and therapeutics available in 2024, previous outbreaks have highlighted significant logistical challenges that exist in managing Ebola outbreaks.
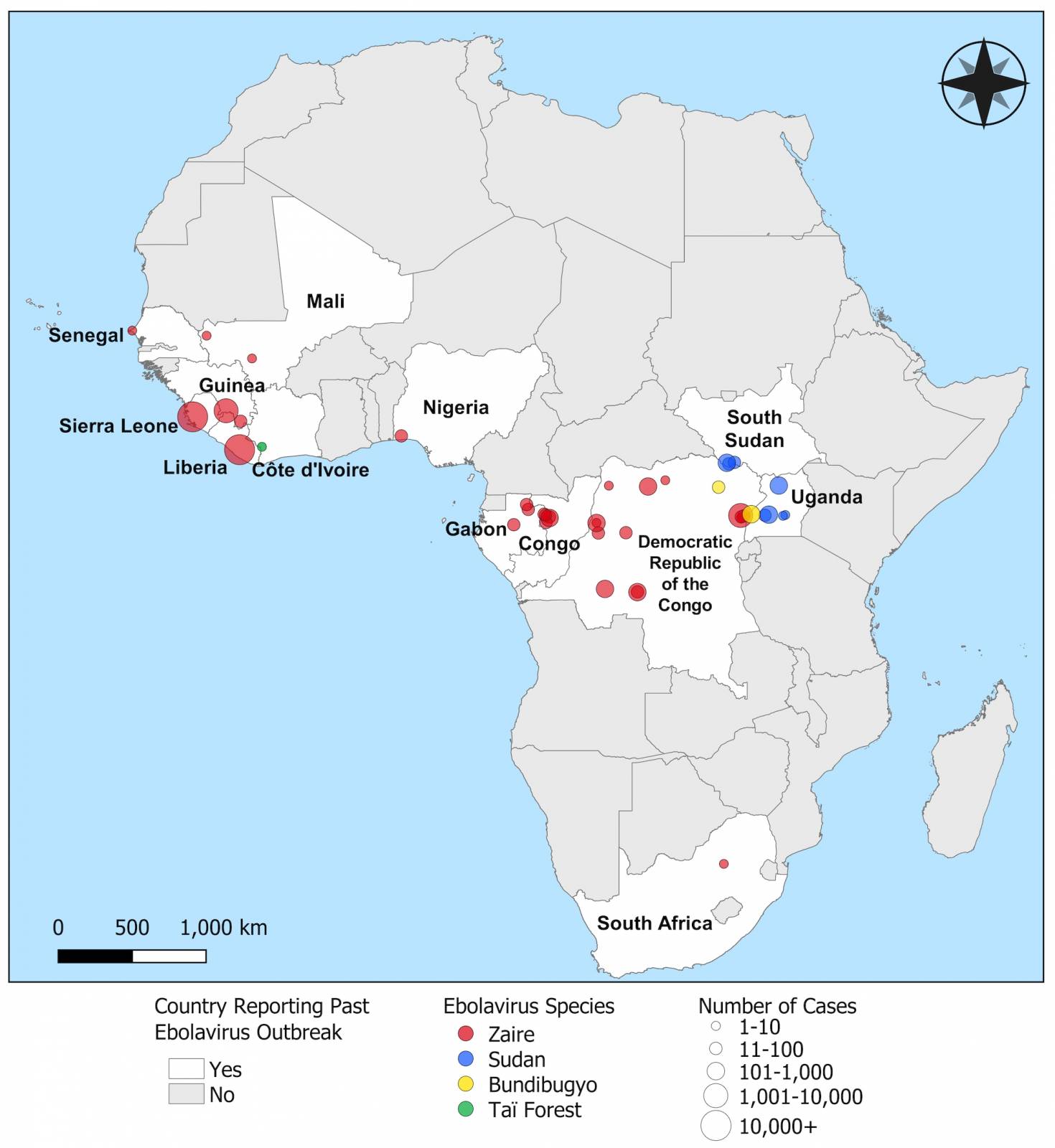
As of October 2024, more than 30 Ebola outbreaks have been reported in Africa. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River in Africa, and the virus's origins remain enigmatic in 2024.
The Islamic Republic of Pakistan is one of two countries left in the world where poliovirus continues to threaten the health and well-being of its 250 million residents.
According to the weekly report published by the Global Polio Eradication Initiative (GPEI), Pakistan confirmed four new wild poliovirus type 1 (WPV1) cases. WPV1 is the only circulating wild poliovirus serotype.
The latest instances of paralysis were in Balochistan, Khyber Pakhtunkhwa, and Sindh provinces.
Furthermore, 50 WPV1-positive environmental samples were recently confirmed from Balochistan, Islamabad, Khyber Pakhtunkhwa, Punjab and Sindh.
As of October 14, 2024, Pakistan has reported 28 cases of WPV1 this year.
Since 1994, the Pakistan Polio Eradication Program has been fighting to end poliovirus infections. Through its efforts, case numbers in Pakistan have declined by up to 99% from the 20,000 cases reported in the early 1990s.
Unfortunately, the WHO reconfirmed in August 2024 that the spread of the poliovirus remained a Public Health Emergency of International Concern.
The U.S. CDC reported in September 2024 that routine immunization coverage with oral polio vaccines (OPV) and inactivated polio vaccine (IPV) in Pakistan has improved in recent years, as IPV protects against paralysis.
However, because it is an inactivated vaccine that does not replicate in the intestinal tract, as does OPV, it does not prevent the spread of poliovirus.
This could partly explain the relatively low number of WPV1 cases reported in the context of widespread WPV1 circulation as evidenced by environmental surveillance.
However, one-half of all WPV1 patients had never received OPV through routine immunization, indicating population immunity gaps. Whenever feasible, vaccination activities need to be synchronized with those of neighboring Afghanistan, says the CDC.
In Africa and Asia, the nOPV2 vaccine has been offered in 2024.
In the United States, the IPV has been offered since 2000, and booster doses are recommended for certain international travelers in 2024.
Next week, the U.S. CDC's Advisory Committee on Immunization Practices (ACIP) vaccine experts and staff will meet in Atlanta, Georgia, to review scientific data and vote on vaccine recommendations.
The agenda for the October 23-24, 2024, meeting includes presentations focused on chikungunya, influenza, pneumococcal, and RSV, but it is not limited to these diseases/vaccines.
Several ACIP votes are planned during the meeting. The vote language linked here is considered 'draft language.'
All ACIP votes and recommendations are not final until the CDC's Director approves them.
Members of the public interested in making an oral public comment are strongly encouraged to submit a request to the CDC no later than October 18, 2024.

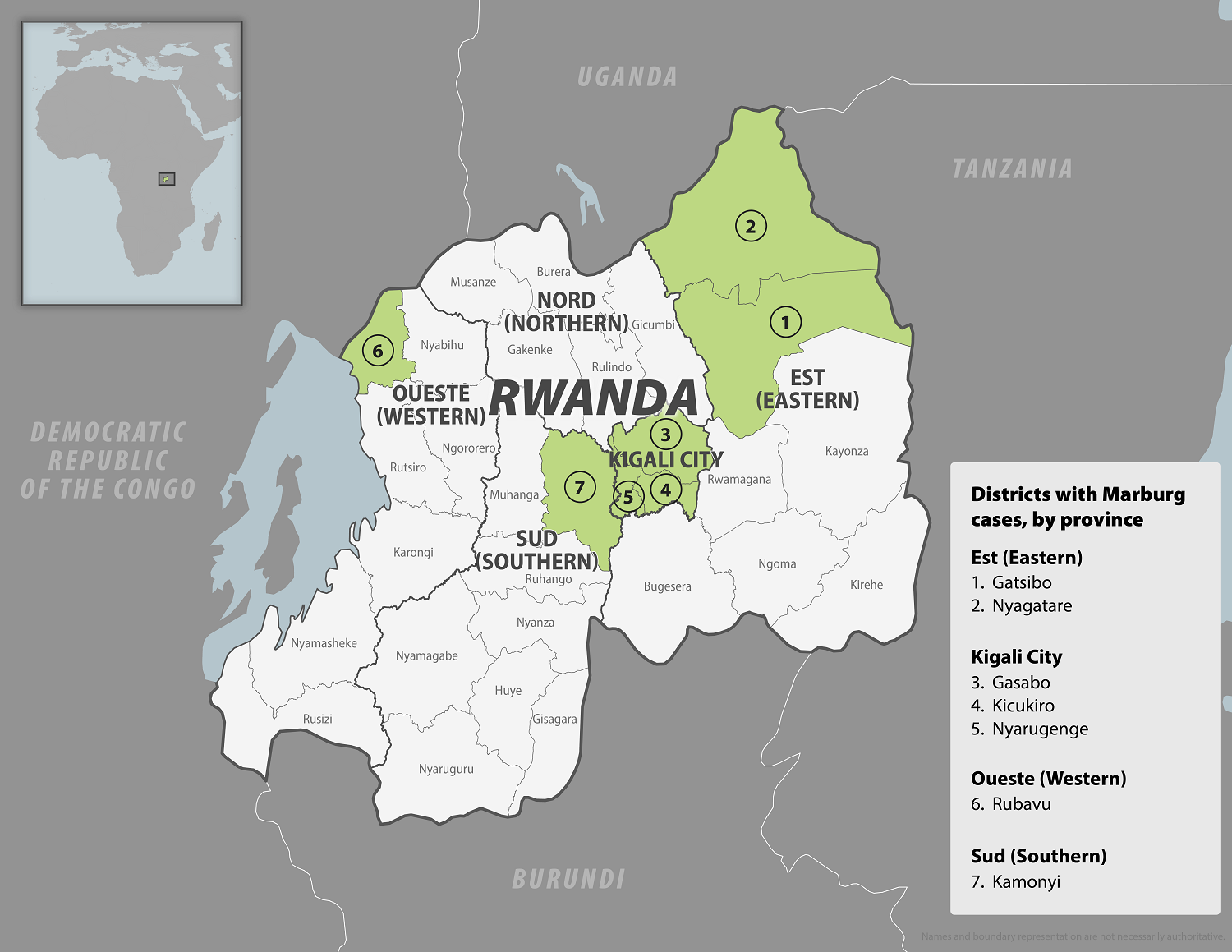
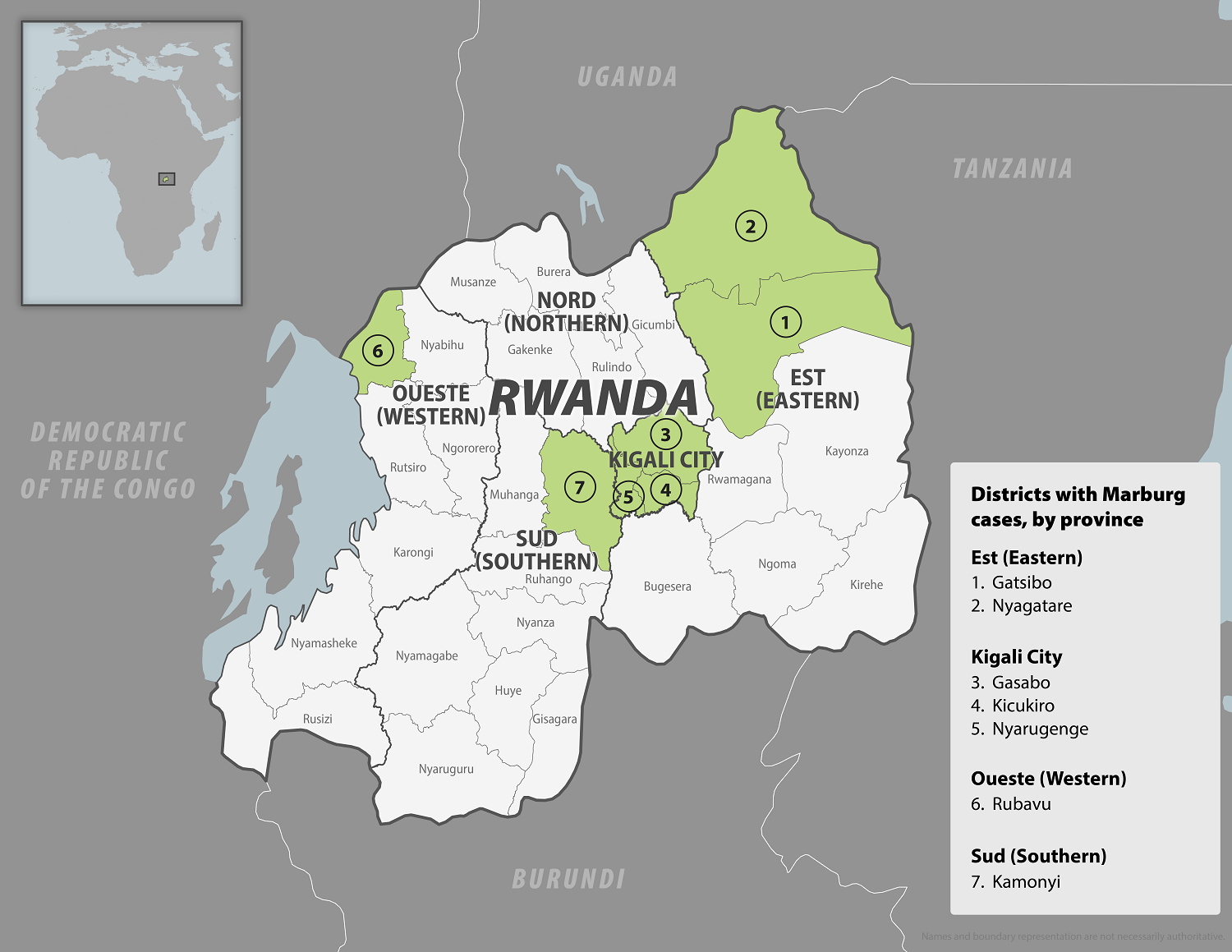
In early October 2024, the Republic of Rwanda began vaccinating frontline health workers in a Phase 2 rapid response open-label clinical trial to combat the reaction to the ongoing Marburg virus disease (MVD) outbreak, which has already claimed 14 lives.
Sabin Vaccine Institute’s single-dose Marburg vaccine candidate was selected to be administered in accordance with the clinical protocol reviewed and approved by Rwandan ethics and regulatory authorities. However, this is not a U.S. FDA-approved vaccine.
As of October 12, 2024, Sabin announced it had delivered approximately 1,700 investigational vaccine doses to Rwanda.
“In an outbreak, every moment counts, and our seamless collaboration with the Rwandan government was key to accelerating the process. On our side, we moved quickly by leveraging our experience with other outbreaks and having vaccine doses and supporting documents ready, thanks to a strong partnership with ReiThera,” says Sabin's CEO Amy Finan in a press release.
Sabin has extensive expertise in advancing vaccines for filoviruses, with two programs currently in Phase 2 clinical trials—one for Marburg and the other for Sudan ebolavirus.
The U.S. government has obligated $235 million to Sabin to advance vaccine research and development against Sudan ebolavirus and MVD.
As of October 13, 2024, other MVD vaccine candidates are conducting clinical research.
Previously, the U.S. CDC announced that people should reconsider nonessential travel to the Republic of Rwanda and that those who arrive in the U.S. may be screened for the virus at certain airports.
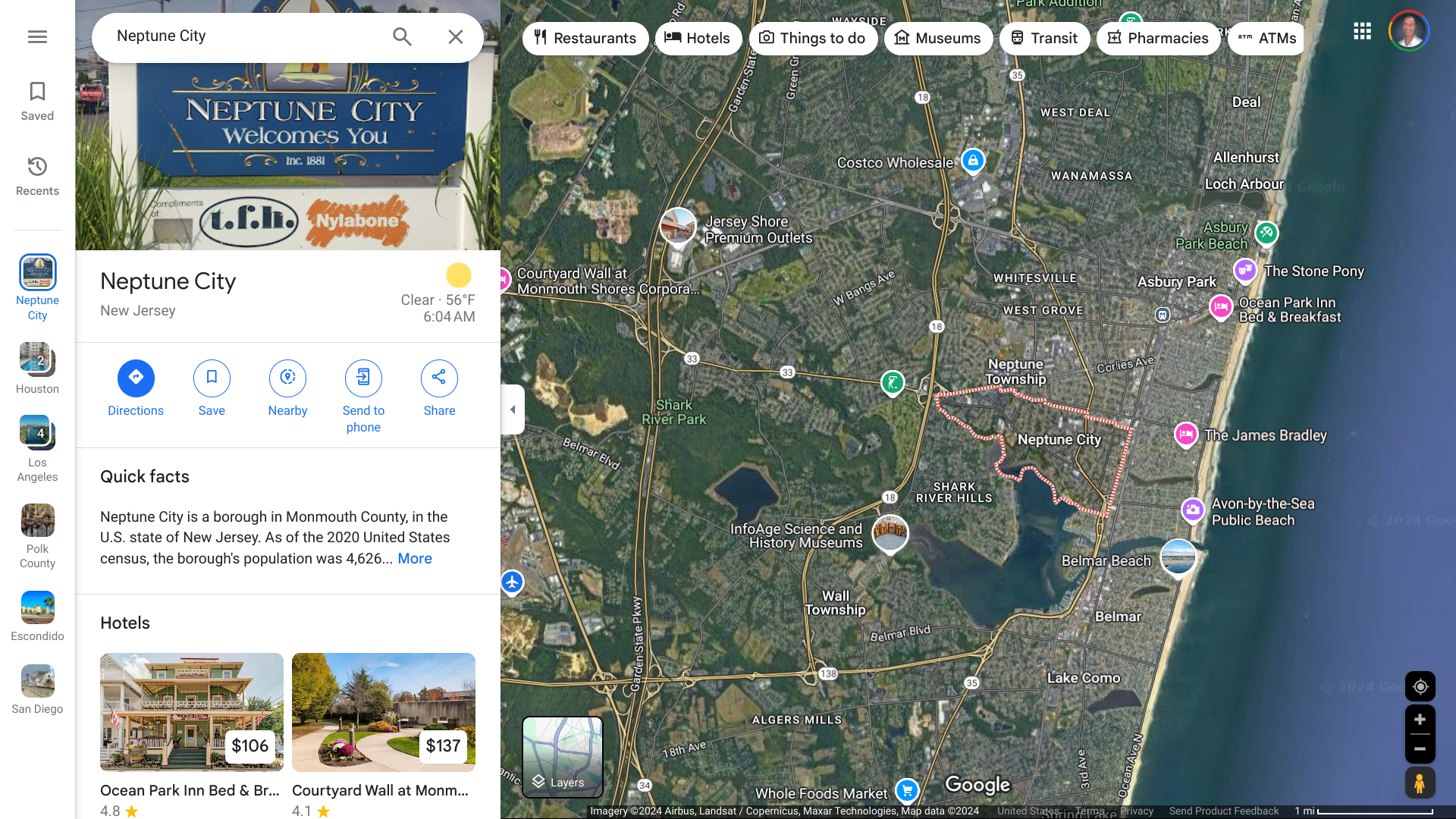
The New Jersey Department of Health (NJDOH) issued an alert regarding a Monmouth County resident who developed measles following recent international travel and visited several locations while infected, including the Jersey Shore University Medical Center in Neptune City.
The NJDOH is working in collaboration with local health officials to identify and notify people who might have been exposed during the time the individual was infectious.
As of October 11, 2024, no additional N.J. cases have been identified. However, secondary measles cases would be expected to occur no later than October 29, 2024.
This is the third confirmed case of measles reported in N.J. and the 267th in the United States this year.
The Department urges all New Jersey residents planning to travel, regardless of destination, to ensure they are current on all routine and travel vaccinations, especially MMR vaccinations. Measles vaccines are offered at clinics and pharmacies in 2024.
On September 24, 2024, the U.S. CDC republished a global Travel Health Notice identifying measles outbreaks in 57 countries.
Dengue is a mosquito-borne viral infection rapidly emerging as a pandemic-prone viral disease across the globe. The World Health Organization (WHO) says the incidence of dengue has increased 30-fold over the last 50 years, especially during rainy seasons.
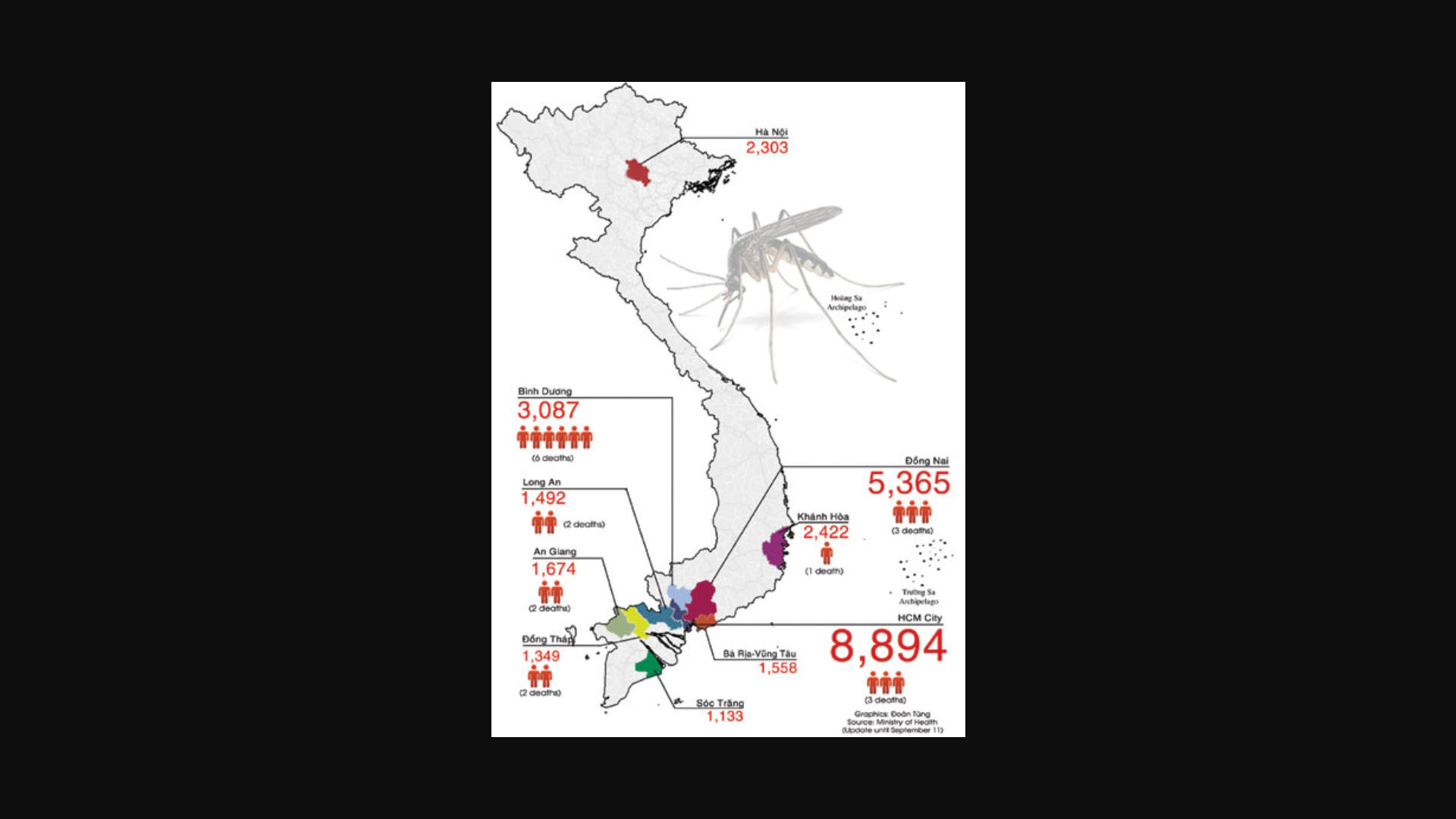
In 2023, over 500,000 dengue cases and 750 deaths were reported from eight countries/territories/areas in the WHO Western Pacific Region, which includes Vietnam.
The WHO reported on October 3, 2024, Vietnam confirmed 76,838 dengue cases, including 12 deaths this year.
According to local news published on September 21, 2024, Vietnam launched its dengue vaccination program in mid-September 2024.
In a media article, Dr. Bach Thi Chinh, Medical Director of VNVC Vaccination System, said that the Ministry of Health approved Takeda's QDENGA dengue vaccine in May 2024 for children from 4 years old and adults.
'The vaccine is particularly effective in preventing reinfection in individuals who have previously contracted dengue fever, which is crucial for Vietnam due to the high prevalence of such cases. Subsequent infections are often more severe than initial ones. Therefore, timely vaccination is essential for safeguarding patients' health and lives.'
This second-generation dengue vaccine will help Vietnam reduce the disease burden and minimize the number of hospitalizations.
Takeda's dengue vaccine is offered in about 40 countries in 2024.
In a rebuttal to recent U.S. government policy, the World Health Organization (WHO) stated, 'At this time, travel and trade restrictions are ineffective and unnecessary for the control of the ongoing outbreak of Marburg virus disease (MVD) in the Republic of Rwanda and are potentially harmful to the affected societies and economies.'
'In addition, travel and trade restrictions may act as a disincentive for rapidly sharing public health data and information with and amongst the global health community, which is critical for informed outbreak response.
The U.S. CDC stated on October 7, 2024, 'Reconsider nonessential travel to Rwanda, which is experiencing an outbreak of MVD.'
Since September 27, 2024, when the Rwanda Ministry of Health confirmed the country's first outbreak of MVD, 61 cases and 14 related deaths have been reported.
As of October 11, 2024, no approved MVD vaccines exist, but experimental vaccines are being tested in Rwanda.