Search API
Since 2023, the U.S. FDA has approved the Chikungunya virus vaccine, which has been deployed in various countries to curtail outbreaks, with exceptional efficacy data reported by multiple studies.
Adding to this positive trend, Valneva SE today reported further positive Phase 3 clinical trial data in adolescents for its single-shot chikungunya virus vaccine, IXCHIQ®. The vaccine showed a sustained 98.3% sero-response rate one year after a single vaccination.
These results support and strengthen the pivotal data previously reported for adolescents (12 to 17 years old), which supported filing for potential label extensions for this age group in the U.S., Europe, and Canada.
Data from this trial are also expected to support the licensure of IXCHIQ® in Brazil, which would be the first potential approval for use in Chikunguna endemic populations.
Juan Carlos Jaramillo, M.D., Valneva's Chief Medical Officer, commented, “These additional adolescent data confirm IXCHIQ®’s ability to induce a robust, long-lasting antibody response in both younger people and adults with a single vaccination."
"Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it’s imperative to ensure the vaccine is available to all age groups and has the potential to offer long-term protection, particularly in low- and middle-income countries where vaccine access is often limited."
"We are now looking forward to the first data in children, which we expect to report imminently.”
Chikungunya outbreaks have been recorded as early as 1824 in India. In 2024, over 425,00 cases and 236 related fatalities were reported in the Region of the Americas.
So far, in 2025, there have been 523 Chikungunya cases in Brazil.
IXCHIQ® is the only licensed Chikungunya vaccine available at travel clinics and pharmacies in the U.S.

The Coalition for Epidemic Preparedness Innovations (CEPI) today announced that Afrigen Biologics aims to develop the first-ever mRNA-based vaccine against Rift Valley fever, supported by a new $6.2 million grant.
Confirmed on January 20, 2025, the researchers will work with the International Vaccine Institute to progress the new vaccine candidate through preclinical development and into Phase I clinical testing in people in either South Africa or another outbreak-affected country on the continent.
If clinical trials are successful, this vaccine could offer a critical new, locally produced tool to help combat this potentially deadly illness, which poses significant risks to human health and livestock.
Dr Richard Hatchett, CEO of CEPI, commented in a press release, “This new research will further strengthen the continent’s future preparedness and response capabilities, thereby enhancing Africa’s vaccine sovereignty and health security.”
First identified in Kenya’s Rift Valley in the 1930s, Rift Valley fever usually occurs in people following direct contact with infected animals, like sheep, goats, and cattle, or bites from infected mosquitoes, says the U.S. CDC.
The disease has also expanded in range in recent years with outbreaks in the Middle East and Indian Ocean islands, hence the need for new Rift Valley fever (RVF) vaccines.
Fortunately, a case of RVF virus spreading from person to person has never been reported.
While the majority of people infected experience mild disease, around 1-2% of those infected can develop the severe hemorrhagic form, which can cause blindness, convulsions, encephalitis, and bleeding and has mortality rates of around 50%.
Although vaccines against RVF have been registered for animals, no vaccines are available or licensed for human use. Therefore, the World Health Organization and the African Centres for Disease Control and Prevention recognize it as a priority target disease.
As of January 4, 2025, the CDC did not report any Rift Valley fever cases in 2024 or 2025.
The Houston Health Department (HHD) recently announced two measles cases associated with international travel. Both adults reside in the same Houston, Texas household and have unknown vaccination statuses.
HHD stated these are the first reported measles cases in Houston since 2018.
Texas experienced a travel-related measles outbreak in 2019, which led to 23 cases.
Measles was officially eliminated from the United States in 2000. However, as of late December 2024, 32 U.S. jurisdictions, led by Illinois and Minnesota, had reported 284 cases, many of which were travel-related.
Internationally, the U.S. Centers for Disease Control and Prevention (CDC) maintains a global Watch-Level 1 Travel Health Notice identifying measles outbreaks in 59 countries last year.
As of January 19, 2025, HHD and the CDC say the most effective way to prevent measles virus infections is to get the measles, mumps, and rubella (MMR) vaccine. Measles vaccination services are offered at most travel clinics and pharmacies.

The Centers for Disease Control and Prevention (CDC) has detected an increase in extensively drug-resistant Shigella infections in the United States over the past few years.
As of January 11, 2025, the U.S. CDC confirmed 296 Shigellosis cases have already been reported in 2025, led by New York (53) and Florida (42).
Last year, the CDC confirmed 20,621 Shigella cases nationwide, led by California (4,365) and New York (2,990).
In Northern Nevada, the Public Health (NNPH) agency identified a Shigellosis outbreak in Reno / Washoe County after a reported influx of new cases and hospitalizations. About 14 cases and nine hospitalizations were reported, although the number of cases is expected to be much higher.
However, there is a low risk of transmission to the general public in 2025.
Shigellosis is an intestinal infection that causes diarrhea, fever, and stomach pain. Shigellosis can be spread by coming into contact with the poop of an infected person, eating or drinking contaminated food or water, or through sexual contact.
According to the CDC, Shigellosis can be challenging to treat, and prevention is critical to reducing the spread of the infection.
As of January 18, 2025, the U.S. FDA has not approved a preventive vaccine. However, a tetravalent bioconjugate vaccine candidate has progressed into phase 2 clinical research.

Despite spending $4 billion annually, the number of malaria cases and deaths has not significantly changed over the past decade, especially in Africa. Last year, the WHO's African Region reported the broadest malaria outbreak burden.
Based on today's U.S. Centers for Disease Control and Prevention (CDC) Travel Health Advisory, health agencies are not optimistic about seeing any improvement in this trend by 2025.
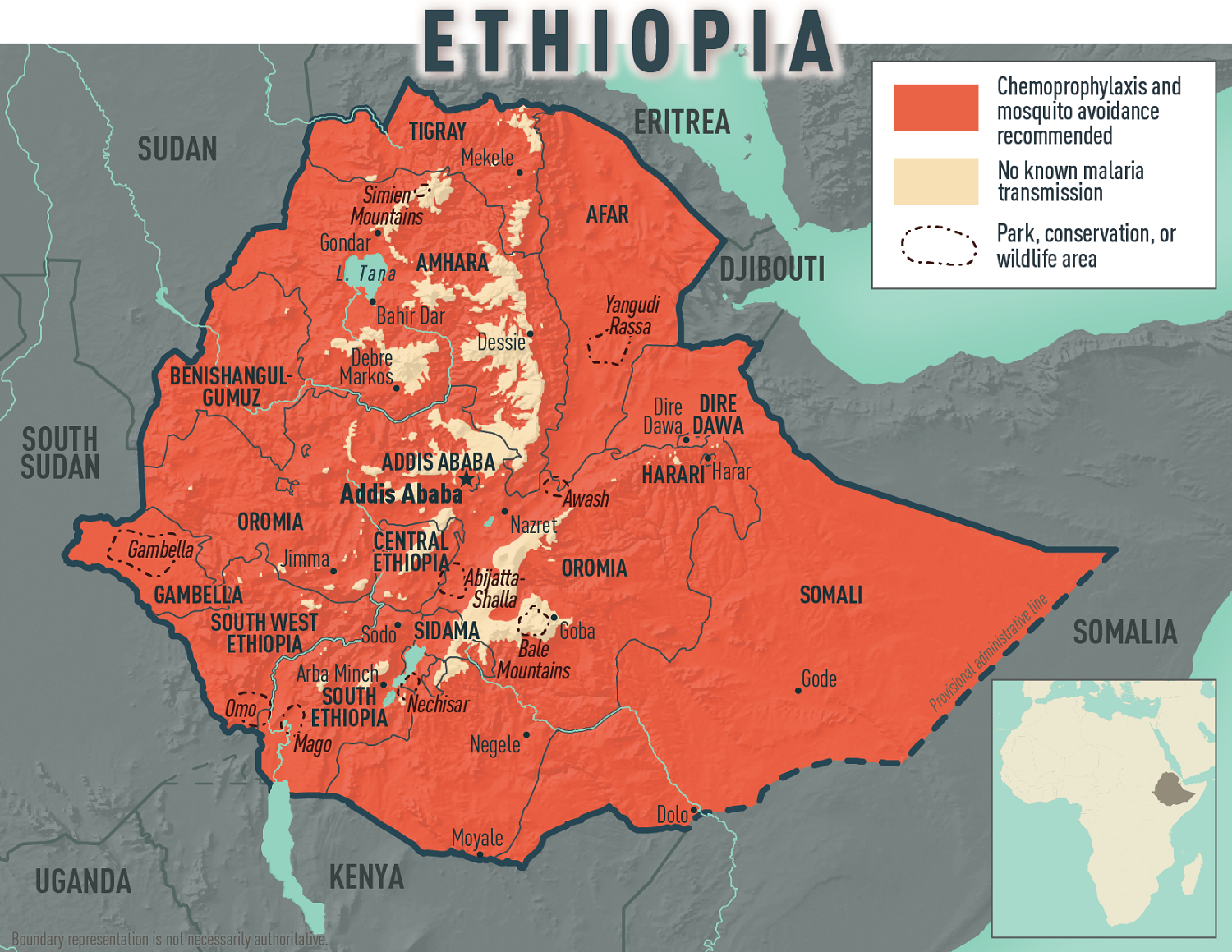
Today, the CDC confirmed an ongoing malaria outbreak in the Federal Democratic Republic of Ethiopia, affecting all 14 country regions. More than 8.4 million malaria cases were reported, the highest number of cases ever reported within a year.
To bolster Ethiopia’s fight against malaria, the United States Government, through the U.S. Agency for International Development, donated 175 computer terminals to the Ethiopian Public Health Institute on January 15, 2025.
Additional U.S. support includes over $27.5 million of antiretroviral supplies, early infant diagnostics, quality assessment panels, lab equipment, and more.
Malaria is a disease caused by a parasite that spreads to humans through the bite of infected mosquitoes, commonly found in Africa.
If you plan to travel to Ethiopia in 2025, the CDC recommends speaking with a travel health expert about which antimalarial drug is best for you. And seek medical care immediately if you develop fever, chills, sweats, headache, vomiting, or body aches during or after travel to Ethiopia.
In 2024, numerous international travelers brought malaria back with them.
As of the week ending November 23, 2024, the CDC confirmed 1,772 malaria cases, mostly among international travelers arriving in New York City (232), Texas, Miami, Florida, and Los Angeles, California.
While malaria vaccines are available in Africa, they are not FDA-approved in the U.S. and remain unavailable in the U.S.
Furthermore, innovative vaccine candidates, such as the RH5.1/Matrix-M malaria vaccine, are proceeding in late-stage clinical trials. Developed at the University of Oxford, this vaccine targets blood-stage malaria, unlike previously approved vaccines that target the pre-erythrocyte stage.
The U.S. Centers for Disease Control and Prevention (CDC) today announced details of a gastrointestinal illness (GI) outbreak on the Silversea Cruises ship Silver Ray.
On January 16, 2025, the CDC reported that 38 guests and five crew members were ill among the 681 people onboard Voyage RA250104016.
This data represents 6.6% of all people onboard the Silver Ray.
The main symptoms of the patients were diarrhea and abdominal cramps. The CDC reported that the causative agent remains unknown.
This incident is the second GI outbreak aboard a cruise ship in 2025.
Last year, the CDC confirmed 18 outbreaks aboard cruise ships, with norovirus as the primary disease agent.
'Norovirus is often a cause of GI outbreaks on cruise ships, but we don't always know the cause of the outbreak when investigations begin, writes the CDC.
A study published in January 2025 identified cruise ship dining areas as priorities for preventing disease outbreaks. However, the probability of airborne infection in a speaking normal condition is low (<3 %).
As of January 2025, norovirus vaccine candidates are conducting clinical research, but none have been U.S. FDA-approved.
For a potential treatment, Travelan®, an orally administered passive immunotherapy, prophylactically reduces the likelihood of contracting travelers' diarrhea. In the U.S., Travelan is sold as a dietary supplement for digestive tract protection.
Note: This article was updated on Jan. 17, 2025, to include a reference link.

The Pan American Health Organization (PAHO), the Government of Argentina, and others today announced a joint effort to facilitate local production and regional access to the 20-valent pneumococcal conjugate vaccine (PCV20).
This vaccine is expected to further protect against severe diseases caused by Streptococcus pneumonia (pneumococcus), responsible for pneumonia, meningitis, and other serious infections, including those linked to antibiotic-resistant strains.
In 2021, 3,345 children under the age of 5 died due to pneumonia and meningitis caused by pneumococcus in Latin America and the Caribbean.
Through this initiative, not only will Argentina benefit from local production of the PCV20 (PREVNAR 20®), but countries across the Region of the Americas will be able to access vaccine doses through PAHO’s Regional Revolving Funds, ensuring more rapid vaccine rollout at competitive prices.
The PCV20 vaccine will be available in Latin America and the Caribbean starting in early 2025. The first doses produced in Argentina are estimated to be available by 2026.
“PAHO is committed to boosting regional production of sustainable, innovative technologies by strengthening existing capacities and our regional purchasing mechanism, the Revolving Fund for Access to Vaccines,” said PAHO Director Jarbas Barbosa in a press release on January 15, 2025.
“This collaboration reflects our dedication to ensuring equitable access to safe, effective vaccines that prevent severe diseases and save lives,” he added.