Search API
The initial Japanese encephalitis virus (JEV) case during the summer of 2025 in Australia's New South Wales (NSW) was likely acquired in the Murrumbidgee region. A previous case was identified in a resident of northern Victoria in January 2025.
On February 15, 2025, NSW Health's Executive Director of Health Protection, Dr. Jeremy McAnulty, said this recent case, which is currently recovering in hospital, will likely have acquired the infection in late December (2024) or early January while on holiday.
"This case, along with recent detections in pigs and mosquitoes in NSW and detections in Victoria and Queensland, highlights the risk of JE virus infection in a large stretch of NSW west of the Great Dividing Range," Dr McAnulty said in a press release.
"It is essential for people who live in or travel to these areas to be aware of the elevated risk and to take precautions against mosquito bites."
"Importantly, there is a safe, effective, and free vaccine to protect against JE which is available to anyone who lives or routinely works in various inland LGAs as well as for people who work in some other high-risk occupations."
In Australia, Valneva SE's IXIARO® —JESPECT® JEV vaccine is available through local General Practitioners, Aboriginal health services, and pharmacists. In 2025, this vaccine will be commercially offered at travel clinics and pharmacies in the United States.
As of February 16, 2025, JEV is the leading cause of viral encephalitis in twenty-four countries in the WHO South-East Asia and Western Pacific Regions, exposing more than 3 billion people to infection risks.
The Quezon City Government, through the City Health Department (QCHD), declared a dengue outbreak today as cases and related fatalities have surged in the city.
From January 1 to February 14, 2025, the City Epidemiology and Surveillance Division of QCHD recorded 1,769 dengue cases, nearly 200% higher than last year. Ten citizens, including eight minors, have already died from the mosquito-transmitted disease.
Fifty-eight percent of the reported cases involve school-aged children (5 to 17 years old).
Mayor Joy Belmonte has mobilized all assets and resources and ensured that programs and services are established and accessible for QCitizens to curb the outbreak.
“Our declaration of a dengue outbreak ensures that we are on top of the situation, and we are doing everything we can to protect our residents from this deadly disease, especially our children,” Mayor Joy Belmonte said in a press release on February 15, 2025.
Quezon City, a tourist top destination located northeast of Manila, is the most populous city in the Republic of the Philippines, with a population of about 2.9 million.
To alert international travelers of their health risks when visiting the Philippines, the U.S. CDC includes this Southeast Asia country in its Global Travel Health Advisory. Other disease risks in 2025 included chikungunya and measles.
Over the past few months, the positive reduction in tuberculosis (TB), the world's leading infectious disease killer, has reversed course in India. Local media has reported that Maharashtra health workers have reported over 24,000 cases as part of the ongoing 100-day campaign under the National TB Elimination Programme (NTEP).
On February 10, 2025, Dr Sandeep Sangle, the Joint Director of Health in Maharashtra, told The Indian Express, "The main aim is to enhance TB detection, improve the efficiency of TB testing and treatment, reduce the mortality rate, and prevent new cases through targeted interventions in the districts."
Previously, the NTEP reported the incidence of TB had been reduced from 237 per 1,00,000 population in 2015 to 195 in 2023, and the mortality rate has decreased from 28 to 22 in the same period.
The World Health Organization (WHO) issued an updated Global TB Report in 2024, revealing that approximately 8.2 million people were newly diagnosed with TB in 2023, the highest number ever recorded by the WHO.
In other United States, TB cases have steadily increased over the past three years. The U.S. CDC reported 8,040 TB cases in 2024, led by California (1,623), New York (901), and Texas (728).
TB is a vaccine-preventable disease, with various versions of the 100-year-old BCG vaccine offered globally.
While the CDC does not list TB in its Travel Health Advisories for India, it includes chikungunya, dengue, and Zika diseases. Of these diseases, the single-dose IXCHIQ® chikungunya vaccine is offered in travel clinics and pharmacies in the U.S., U.K., and Europe in 2025.
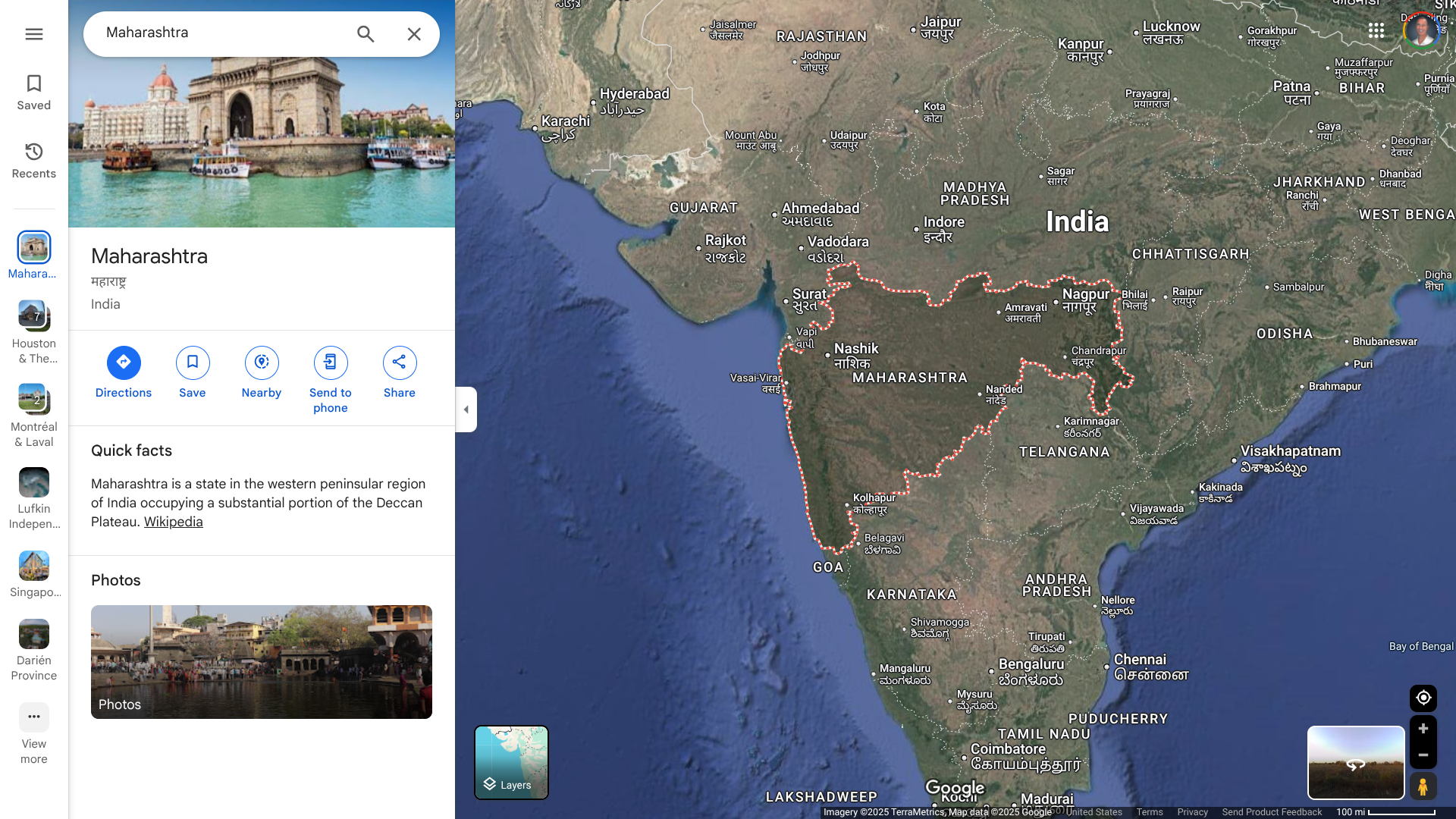
A recent study confirms that human papillomavirus (HPV) vaccination prevents the development of invasive cervical cancer, regardless of dosage.
Published in the Journal of the National Cancer Institute on January 22, 2025, this analysis concluded that even one or two doses one month apart confer benefit if given at 12-13 years of age. At older ages, three HPV doses are required for statistically significant vaccine effectiveness.
No cases of invasive cancer were recorded in women immunized at 12 or 13 years of age, irrespective of the number of doses.
Women vaccinated at 14 to 22 years of age and given three doses of the bivalent vaccine showed a significant reduction in incidence compared with all unvaccinated women (3.2/100 000 [95% confidence interval (CI) = 2.1 to 4.6] vs. 8.4 [95% CI = 7.2 to 9.6]).
Unadjusted incidence was significantly higher in women from most deprived (Scottish Index of Multiple Deprivation 1) than least deprived (Scottish Index of Multiple Deprivation 5) areas (10.1/100 000 [95% CI = 7.8 to 12.8] vs 3.9 [95% CI = 2.6 to 5.7]).
Women from the most deprived areas showed a significant reduction in incidence following three doses of vaccine (13.1/100 000 [95% CI = 9.95 to 16.9] vs 2.29 [95% CI = 0.62 to 5.86]).
Dr. Kirsty Roy, Consultant in Health Protection, Public Health Scotland, and co-author of this encouraging study, said in a press release, "This study involves every woman in Scotland who is eligible for the cervical cancer screening program and demonstrates the impact of the HPV vaccine in preventing cervical cancer."
"It shows how effective the HPV vaccine is as there have been no cervical cancer cases to date in fully vaccinated women who were given their first dose at age 12-13 years.
"Vaccination against HPV is shown to be effective in preventing cervical cancer, and along with regular screening for early detection and treatment, it is possible to make cervical cancer a rare disease."
In the United States, Merck's GARDASIL 9® vaccine has been found to protect women and men ages 9 to 45 against diseases caused by nine types of HPV. GARDSIL is generally available at clinics and pharmacies in the U.S.
The state of Texas may soon be added to the U.S. Centers for Disease Control and Prevention (CDC) travel advisory list of areas reporting measles outbreaks in 2025.
As of February 2025, the Texas Department of State Health Services (DSHS) has confirmed 50 measles cases in five counties this year. Most of these cases are unvaccinated children.
In the South Plains Region of western Texas, 48 cases have been identified in Gaines County (42), followed by Terry County (3), Yoakum (2), and Lynn counties (1).
In late January 2025, the Houston Health Department announced two measles cases.
DSHS said these are the first measles cases since 2023. Unfortunately, due to the highly contagious nature of this virus, additional cases are likely to occur in 2025.
Furthermore, the New Mexico Department of Health reported three cases just west of these Texas cases in February 2025.
Since measles is a vaccine-preventable disease, most community pharmacies in Texas offer the MMR vaccine.

A recent study by the Finnish Institute for Health and Welfare (THL) shows that the Zoonotic Influenza Vaccine Seqirus injektioneste protects people against the disease caused by the currently circulating avian influenza viruses.
Since the summer of 2024, avian influenza vaccinations have been offered to occupational groups in the Republic of Finland at increased risk of the disease, such as those working with fur animals and poultry. They were offered Seqirus's vaccine, which the European Medicines Agency authorized.
Announced on February 13, 2025, the study showed that the vaccine-induced neutralizing antibodies identified the vaccine virus and the avian influenza viruses that caused the outbreaks on fur farms in Finland in 2023 and on dairy farms in the United States in 2024.
About half of the previously unvaccinated individuals received one vaccine dose, which induced antibody levels that were estimated to protect against the disease caused by the avian influenza virus. Two doses induced protective antibody levels in the majority. The results suggest that the vaccine protects against currently circulating avian influenza viruses.
In the United States, the FDA has authorized one avian influenza vaccine, and the government has funded various vaccine candidates.
These 'bird flu' vaccines are not commercially available in the U.S.
With the increased international travel expected this year, an unwelcomed health issue may interrupt these trips over the next decade. Travelers' diarrhea (TD) is the most predictable travel-related illness.
The U.S. CDC says TB attack rates range from 30%-70% of travelers during two weeks, depending on the destination and season of travel.
According to a new report, the traveler’s diarrhea market may grow by 5.26% between 2025 and 2035. The major markets for traveler’s diarrhea include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan.
Published on February 13, 2025, IMARC Group expects innovations in traveler’s diarrhea treatment to sustain the market's growth. Developing new treatments and pharmacologic agents to treat the illness reduces recovery time and concerns about antibiotic resistance.
These innovations include, but are not limited to, the emerging popularity of oral hydration formulas, which travelers can embrace. These formulas can optimize fluid absorption in the intestines, thereby quickly replenishing electrolytes in the body.
While clinically different from TD, Norovirus is a commonly reported cause of diarrhea among travelers in confined spaces, such as on cruise ships. The risk for infection is present anywhere food is prepared unsanitaryly and can be contaminated or where drinking water is inadequately treated. Contaminated ice has also been implicated in outbreaks.
Recently, a gastrointestinal illness outbreak on a cruise ship affected 7.4% of its passengers.
With the rapid expansion of the cruise industry, future Norovirus outbreaks can be expected.
For example, the Port of Galveston recorded about 3.4 million passenger movements on over 380 voyages last year. Once the island's fourth cruise terminal opens, that number could surpass 400.
While TD products are available at travel clinics and pharmacies in 2025, no Norovirus vaccines are approved.

According to disappointing news released today, the world must wait for an approved vaccine that prevents invasive E. coli disease (IED).
Sanofi announced today that a scheduled review of the E.mbrace phase 3 clinical study conducted by an independent data monitoring committee (IDMC) determined that Sanofi and Johnson & Johnson's vaccine candidate for extraintestinal pathogenic E. coli was not sufficiently effective at preventing IED compared to placebo.
As a result of the IDMC's determination, the E.mbrace study is being discontinued.
The study, initiated in June 2021, enrolled older adults with a history of urinary tract infections (UTIs) in the past two years. It was conducted at over 250 sites across five continents. Janssen Research & Development, LLC, is the trial sponsor and responsible party and will continue appropriate safety follow-up for the currently enrolled participants.
Jean-François Toussaint, Sanofi's Global Head of Research and Development Vaccines, commented in a press release on February 13, 2025, "E. coli sepsis is a devastating disease, and no preventative measures are available to date."
In October 2023, Sanofi agreed with Janssen Pharmaceuticals, Inc. (Janssen), a Johnson & Johnson company, to develop and commercialize the vaccine candidate. As a result of the discontinuation, Sanofi has recorded an impairment charge before tax of $250 million in the Q4 2024 results.
UTIs are among the most common bacterial infections, many of which are caused by uropathogenic E. coli. Still, less common pathogens, such as Enterococcus faecalis and other enterococci, can cause infections by infecting an abnormal or catheterized urinary tract.
UTIs are more common in females because their urethras are shorter, making it easier for bacteria to enter the urinary tract. Moreover, younger people also suffer from UTIs.
As of 2025, the U.S. Centers for Disease Control and Prevention says most UTIs can be treated with antibiotics prescribed by a healthcare professional.
However, international travelers seeking access to a non-FDA-approved UTI vaccine (Uromune™, MV140) outside of the USA can submit an appointment request using this Vax-Before-Travel link.