Search API
The U.S. CDC published today the Emerging Infectious Diseases, Early Release, Volume 30, Number 4—April 2024, that concluded where feasible, vaccination against hepatitis A, meningococcal disease (IMD), and mpox should be encouraged among at-risk groups and offered along with program services that target those groups.
On March 18, 2024, these CDC researchers wrote, 'We provide a descriptive, cross-sectional analysis of the concurrent outbreaks of hepatitis A and IMD in Florida in the context of an ongoing global mpox epidemic that also is disproportionally affecting MSM.'
'Through this analysis, we attempted to identify common and distinct features of each outbreak and synergistic factors that might have affected disease progression and control.'
'We observed a high percentage of concurrent HIV infection among hepatitis A, IMD, and most notably, mpox case-patients.'
The U.S. Food and Drug Administration has approved vaccines that can prevent certain sexually transmitted diseases (STDs) caused by infections from bacteria, viruses, or parasites. Various STD vaccines are available at health clinics and pharmacies in the U.S.

The Bill & Melinda Gates Medical Research Institute today announced that a Phase 3 clinical trial to assess the efficacy of the M72/AS01E tuberculosis (TB) vaccine candidate is now underway in South Africa.
At full capacity, the trial will include up to 20,000 participants, including people living with HIV, at up to 60 trial sites in seven countries.
If shown to be well-tolerated and effective, M72/AS01E could potentially become the first vaccine to help prevent pulmonary TB in adolescents and adults, the most common form of the disease.
Furthermore, M72/AS01E would be the first new TB vaccine in over a century.
While TB is one of the world’s deadliest infectious diseases, the only available vaccine is Bacille Calmette-Guerin (BCG), which dates back to 1921.
BCG vaccines initially targeted against TB, tuberculosis meningitis, and non-specific protective effects against respiratory tract infections and certain cancers.
Various reports indicate that the BCG vaccine offers inadequate protection for adolescents and adults against the pulmonary form of the disease, which is primarily responsible for transmitting the TB bacterium.

Merck today announced positive data from multiple Phase 3 studies evaluating V116, the company’s investigational, adult-specific 21-valent pneumococcal conjugate vaccine.
Across the clinical studies presented at the 13th Meeting of the International Society of Pneumonia and Pneumococcal Diseases in Cape Town, South Africa, on March 19, 2024, V116 was shown to be immunogenic for all 21 serotypes covered by the vaccine in a variety of adult populations, including those who had not previously received a pneumococcal vaccine (pneumococcal vaccine-naïve), those who had previously received a pneumococcal vaccine (pneumococcal vaccine-experienced) and those with an increased risk of pneumococcal disease, including people living with human immunodeficiency virus.
In all STRIDE studies presented at the meeting, V116 also elicited higher immune responses than the studied comparators for the serotypes unique to V116.
Several studies presented today were included in the filing submission to the U.S. Food and Drug Administration (FDA). The FDA granted V116 priority review with a Prescription Drug User Fee Act of June 17, 2024.
If approved by the FDA, V116 would be the first pneumococcal conjugate vaccine specifically designed for adults.
The journal Vaccines recently published a Short Communication that presented a benefit–risk assessment for the Novavax COVID-19 protein-based vaccine (NVX-CoV2373).
Published on March 16, 2024, this analysis used data on myocarditis/pericarditis cases observed in the NVX-CoV2373 clinical studies, real-world data of mRNA COVID vaccine effectiveness against predominant SARS-CoV-2 strains in early 2023, and recent COVID-19 burden of disease data from the U.S.
The benefits of NVX-CoV2373 vaccination were estimated as the number of COVID-19 cases, hospitalizations, and deaths prevented. The risks of myocarditis/pericarditis cases and related hospitalizations and deaths occurring within seven days of vaccination were also estimated.
In our analysis, vaccination with NVX-CoV2373, per 100,000 vaccinated, prevented an estimated 1805 COVID-19 cases, compared with an estimated 5.3 excess myocarditis/pericarditis cases.
The number of COVID-19 hospitalizations and deaths prevented were also greater than vaccine-associated myocarditis/pericarditis hospitalizations and deaths.
Our analysis indicates a positive benefit–risk balance for NVX-CoV2373, concluded these researchers.
In October 2023, the U.S. Food and Drug Administration amended the EUA of the Novavax COVID-19 Vaccine, Adjuvanted (Nuvaxovid™ XBB.1.5) for use in individuals 12 and older, to include the 2023-2024 formula.
In the U.S., the current Novavax COVID‑19 Vaccine is available at certain pharmacies, including Costco, CVS Pharmacy, Giant, Publix, Rite Aid, and Stop & Shop.

The U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization say the increased number of measles outbreaks is a global threat in 2024.
As of March 14, 2024, a total of 58 measles cases were reported by 17 U.S. jurisdictions: Arizona, California, Florida, Georgia, Illinois, Indiana, Louisiana, Maryland, Michigan, Minnesota, Missouri, New Jersey, New York City, Ohio, Pennsylvania, Virginia, and Washington.
In 2023, a total of 58 measles cases were reported by 20 U.S. jurisdictions.
In 2024, the Chicago Department of Public Health (CDPH) confirmed twelve measles cases, including an active outbreak at a local shelter in Pilsen. CDPH is coordinating a comprehensive, city-wide response to the first measles cases in Chicago in five years.
CDPH is encouraging all new arrivals to Chicago and every Chicagoan to get the measles-mumps-rubella (MMR) vaccine if they haven't already done so.
The vast majority of Chicagoans are vaccinated against measles and therefore not at high risk," said CDPH Commissioner Olusimbo 'Simbo' Ige, MD, MPH, in a press release on March 12, 2024.
"But those who are unvaccinated need to take precautions. If they're exposed, quarantine immediately and connect with their healthcare provider. Above all else, get vaccinated so you, too, can be protected from this virus."
In the U.S., MMR vaccines are available at most community pharmacies as of March 18, 2024.
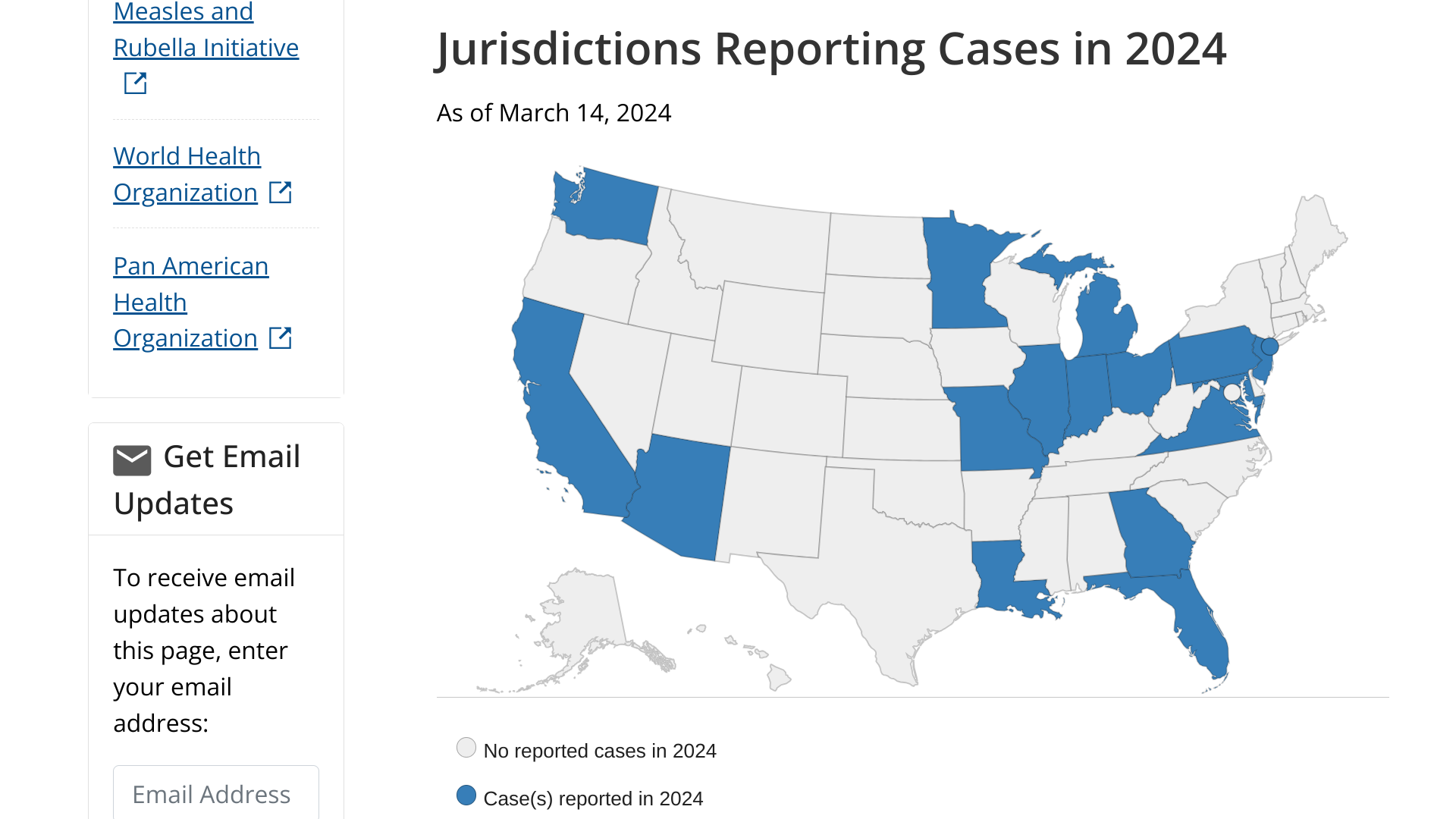
The U.S. National Center for Health Statistics (NCHS) Mortality Surveillance data available on March 14, 2024, indicates that 0.7% of the deaths that occurred during the week ending March 9, 2024 (Week 10) were due to influenza.
This percentage increased (≥ 0.1 percentage point change) compared to last week's data.
NCHS's data also confirmed that 13 influenza-associated pediatric deaths were reported to the U.S. CDC during Week 10. The total number of pediatric deaths for the 2023-2024 flu season is now 116.
As of March 17, 2024, the CDC continues to recommend that everyone six months and older get an annual flu vaccine as long as influenza viruses are spreading. Vaccination can still provide benefits this flu season.
Various egg, cell, and nasal flu shots remain available at most community pharmacies in the U.S.
The composition of flu vaccines is reviewed annually, and new flu vaccines are manufactured each year. For the 2024-2025 flu season, many countries are transitioning to trivalent flu vaccines.