Search API
A vaccine candidate for control of the cytomegalovirus (“CMV”) in patients undergoing liver transplantation dosed its initial patient in a multi-center, placebo-controlled, randomized Phase 2 clinical study.
Initially developed by the City of Hope, Triplex was exclusively licensed to Helocyte.
Triplex is a universal (non-HLA-restricted) recombinant Modified Vaccinia Ankara viral vector vaccine engineered to induce a robust and durable virus-specific T cell response to three immuno-dominant proteins [UL83 (pp65), UL123 (IE1), UL122 (IE2)] linked to CMV complications in the post-transplant setting.
The trial is funded by a grant from the U.S.S NIH’s National Institute of Allergy and Infectious Diseases to the University of Washington Seattle. This grant has provided $9 million to date, with an estimated additional $12 million over the next four years in support of the Phase 2 clinical trial.
Ajit Limaye, M.D., Professor of Medicine and Director of the Solid Organ Transplant Infectious Disease Program at the University of Washington and Principal Investigator of the “CMV vaccine in Orthotopic Liver Transplant” trial, said in a press release on May 14, 2024, “There remains a significant unmet medical need to develop new therapies that can reduce the frequency and severity of CMV events in the organ transplant setting, where CMV continues to present life-threatening complications that directly impact patient outcomes and survival.”
According to the U.S. CDC, CMV is a common virus for people of all ages.
In the U.S., nearly one in three children is already infected with CMV by age five. About 1 out of 200 babies are born with congenital CMV.
And over half of adults have been infected with CMV by age 40.
Once CMV is in a person’s body, it stays there for life and can reactivate. According to the CDC, most people with CMV infection have no symptoms and aren’t aware that they have been infected.
Helocyte is a clinical-stage company developing novel immunotherapies to prevent and treat cancer and infectious diseases, including CMV and HIV.

Although several countries have adopted a single-dose human papillomavirus (HPV) vaccination strategy, many other countries, such as the United States, continue to include multiple doses in their vaccination programs.
As of May 2024, six vaccines are authorized globally to protect males and females against cancers caused by HPV.
According to an article published by the Lancet Infectious Diseases on May 8, 2024, there are ethical reasons to transition to a single-dose strategy.
These scientists discuss how a single-dose HPV vaccination strategy advances equity in three dimensions: vaccine equity, health equity, and gender equity.
Adopting a single-dose strategy eases pressure on vaccine supply, lowers program costs, and is easier to distribute.
This change facilitates vaccine procurement and implementation programs (contributing to vaccine equity) and reaching hard-to-reach people or populations (contributing to health equity).
A lower number of cases of HPV-related diseases that stem from greater vaccine distribution reduces the burden on women, who are at a higher risk of HPV-related disease or who act as caregivers, which prevents them from accessing opportunities that contribute to their empowerment (contributing to gender equity).
Thus, these scientists wrote that pursuing the single-dose HPV vaccination program strategy is ethically desirable.
In April 2022, WHO's Strategic Advisory Group of Experts on Immunization concluded that a single-dose HPV vaccine delivers virus protection comparable to 2-dose schedules.
In the U.S., the Centers for Disease Control and Prevention (CDC) HPV vaccination has been recommended for women since 2006 and for men since 2011. Current CDC HPV vaccination schedules were updated in 2023.

Mark Suzman, the CEO of the Bill & Melinda Gates Foundation, published a change of leadership message today.
On May 13, 2024, Suzman wrote, 'I am writing to share some important news. Melinda French Gates has decided to resign from her role as co-chair of the foundation. Her last day of work at the foundation will be June 7, 2024.
Melinda cares deeply about the foundation and is extremely proud of all of you and the work you do every day to help millions of people live better lives. She made this decision, after considerable reflection, based on how she wants to spend the next chapter of her philanthropy.
Melinda has new ideas about the role she wants to play in improving the lives of women and families in the U.S. and worldwide.'
The entire unedited message is posted at this Gates Foundation link.
And listen to the Gates Foundation's $8.6 billion budget 2024 Annual Letter, read by Suzman.
The Gates Foundation has been a global leader in expanding access to vaccines.
For example, In 1988, when the Global Polio Eradication Initiative (GPEI) was launched, polio was present in more than 125 countries and paralyzed about 1,000 children per day.
Thanks to the Gates immunization efforts, nearly 3 billion children have been immunized, and the incidence of polio has decreased by 99%.
Gate and GPEI have recently successfully deployed the nOPV2 vaccine to over 1 billion people.

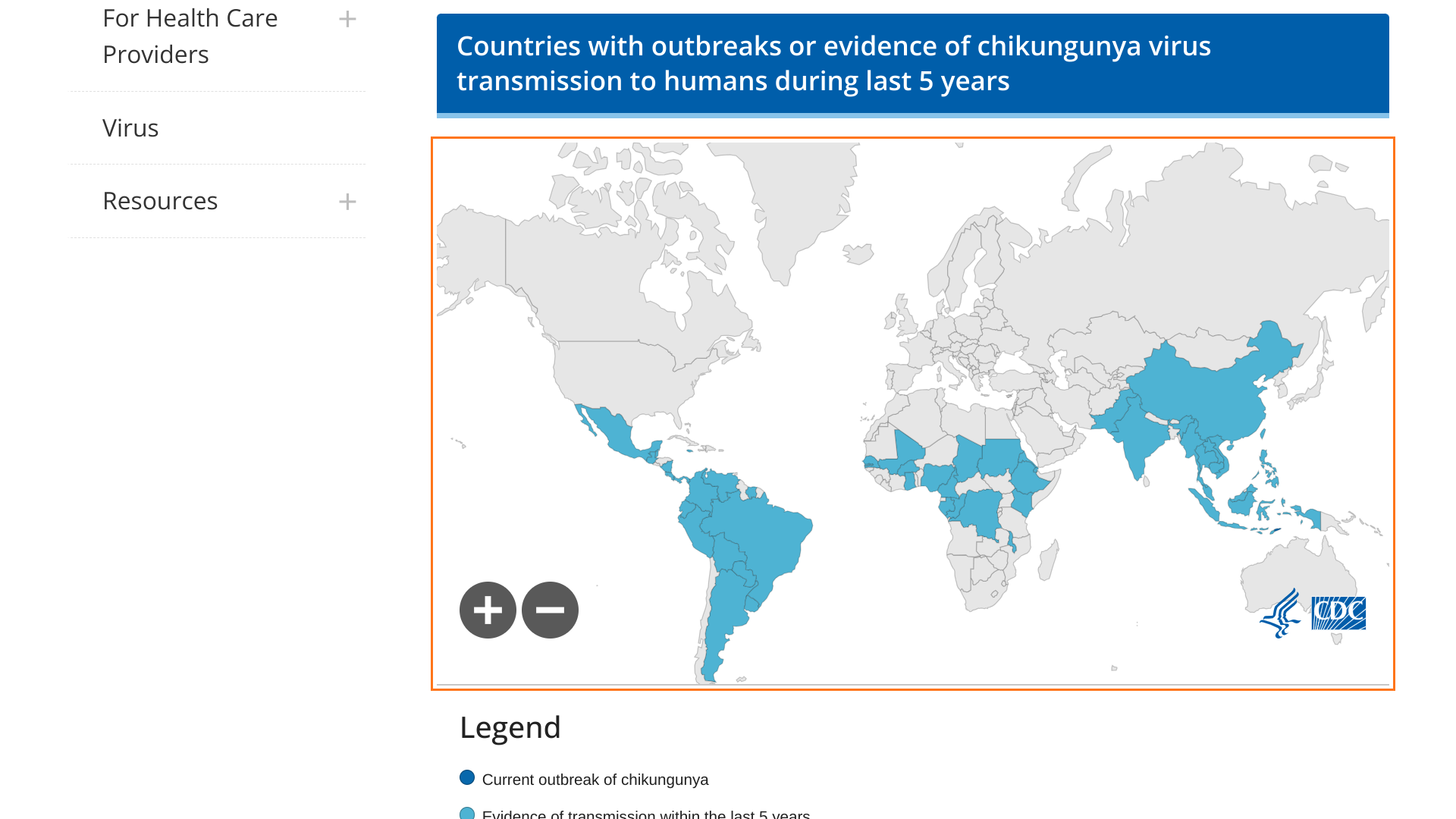
The world's first and only licensed chikungunya vaccine today presented further positive pivotal Phase 3 data in adolescents.
On May 13, 2024, Valneva SE announced following the initial analysis up to Day 29 post-vaccination, the most recent analysis of study VLA1553-321 evaluated the safety and immunogenicity six months after vaccination with a single dose of the chikungunya virus (CHIKV) vaccine IXCHIQ®.
The Day 180 results confirm the initial positive immunogenicity and safety data Valneva reported previously and are intended to support filing for potential label extension for use in adolescents aged 12 to 17 years.
The data are also expected to support the licensure of IXCHIQ® in Brazil, which would be the first potential approval for use in endemic populations.
The U.S. FDA approved IXCHIQ in November 2023, and the Centers for Disease Control and Prevention (CDC) recently adopted the Advisory Committee on Immunization Practices' recommendations on the use of the vaccine in the U.S.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, said in a press release, "We are highly encouraged by these data, as they reinforce the strong immunity and safety observed in adults and the elderly, upon which FDA approval was granted."
"Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it's imperative to ensure the vaccine is available to all age groups. This broader accessibility can help provide protection and mitigate the burden of this debilitating illness."
Three marketing applications are under review by the European Medicines Agency, Health Canada, and the Brazilian Health Regulatory Agency, with potential approvals in 2024.
The CDC recently issued an updated Level 2 Travel Health Advisory confirming chikungunya vaccination is recommended for adults traveling to a destination with a current CHIKV outbreak.
Developed by Chengdu Weisjin Biomedical Technology Co., Ltd. (Wesjin Biotech), WGc-043 is an mRNA therapeutic cancer vaccine that recently received IND approval from the U.S. Food and Drug Administration (FDA).
According to public information from the FDA, WGc-043 Injection has been approved for two categories of indications: one is for adult patients with Epstein-Barr virus-positive advanced solid tumors who have undergone second-line systemic treatment.
The second indication is for adult patients with relapsed or refractory virus-positive hematoma.
This achievement, announced on May 9, 2024, marks the world's first approval of an Epstein-Barr virus (EBV)- related mRNA therapeutic cancer vaccine.
Once successfully launched, WGc-043 will provide a new treatment option for patients with advanced EB virus-positive solid tumors and hematologic malignancies.
EB virus is highly correlated with more than ten malignancies, including nasopharyngeal carcinoma, natural killer T-cell lymphoma, gastric cancer, lung cancer, liver cancer, esophageal cancer, breast cancer, cervical cancer, and autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus.
According to a press release on May 10, 2024, WGc-043 shows promising efficacy, low toxicity, broad applicability, efficient scalability, and cost-effectiveness.
The company says that WGc-043 has already completed investigator-initiated trials, demonstrating superior safety and efficacy compared to other publicly available mRNA therapeutic cancer vaccines.
Specifically, the technical features of WGc-043 also include the antigen being the most broad-spectrum and safe protein sequence.
The originally designed immune enhancer (IE) is introduced into the mRNA molecule, and the mRNA delivery carrier is independently developed and obtained
A new type of LNP authorized by US and European patents (the safety of this LNP: has been verified in clinical trials of 3 varieties).
These designs enable WGc-043 to activate the patient's own anti-tumor immunity and generate tumor-killing cytotoxic T cells, antigen-specific antibodies, and memory T cells in the body, which is equivalent to CAR-T. The combined anti-tumor effect of monoclonal antibodies can also prevent tumor recurrence, have more efficient anti-cancer effects, and be superiorly safe.
Weisjin Biotechnology has filed over 60 invention patents, including the patent for ionizable lipids, which has been authorized by China, the United States, Europe, and other countries and regions.
As of May 12, 2024, no date has been announced regarding WGc-043's availability in the United States.

Moderna, Inc., today announced that the U.S. Food and Drug Administration (FDA) has notified it that due to administrative constraints, the agency does not expect to complete its review of the Biologics License Application for mRNA-1345, Moderna's investigational respiratory syncytial virus (RSV) vaccine, by the previously communicated Prescription Drug User Fee Act date of May 12, 2024.
The FDA informed Moderna on May 10, 2024, that it is working to conclude its review of mRNA-1345 by the end of May 2024.
The FDA has not informed Moderna of any issues related to vaccine safety, efficacy, or quality that would prevent the approval of mRNA-1345.
The U.S. CDC reported today that seasonal influenza activity continues to decline nationally, with outpatient respiratory illness stable and below baseline for the fifth consecutive week.
As of Week #18, the CDC says that flu viruses are among the several contributing factors to respiratory disease activity. To help people understand their health risks, the CDC provides updated, integrated information about influenza, RSV, and other disease activity every week.
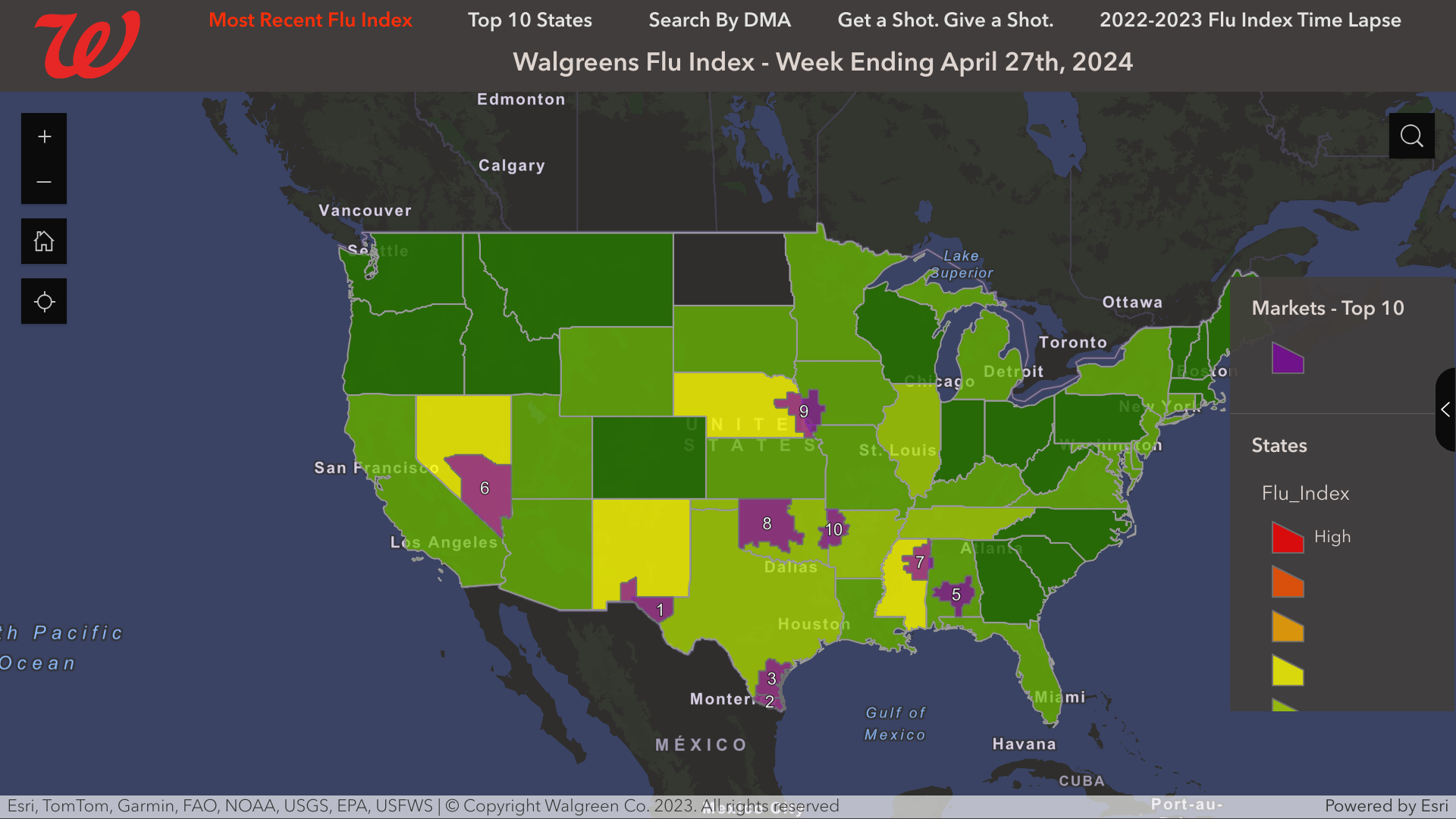
From a local perspective, the Walgreens Flu Index recently identified three Texas cities that led the USA with influenza activity in late April 2024.
These cities are:
- El Paso, Texas (Las Cruces, N.M.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Corpus Christi, Texas
The CDC also disclosed that about 63 million flu shots have been administered at pharmacies and physician offices this flu season, which indicates about 6% fewer flu shots administered than last season.
These vaccines remain available at most pharmacies in the U.S.