About Us
Vax-Before-Travel
Vax-Before-Travel (VBT) is a publisher of international vaccine information that empowers people to make informed immunization decisions before traveling abroad. Throughout 2025, VBT published fact-checked information on local disease risks and related travel vaccines.
"We believe international travelers want to learn about disease risks and vaccine options before their next vacation or business trip," writes Don Hackett, VBT publisher. 'This philosophy reduces disease risks by minimizing the under-use, over-use, and misuse of vaccines.'
When traveling internationally to countries like Costa Rica or within the United States, such as Florida, Vax-Before-Travel's continuously updated information empowers you to 'Know Before You Go.'
Vax-Before-Travel Mission
Vax-Before-Travel publishes vaccine information that empowers travelers to live life to the fullest.
Vax-Before-Travel Content
Vax-Before-Travel's news team stands by the information we publish; if it's incorrect, we will change it as quickly as possible.
Recent studies have highlighted the value that VBT's news brings to people. Key findings from a study published in October 2025 found 45% of AI-produced answers had at least one significant issue from from ChatGPT, Copilot, Gemini, and Perplexity against key criteria, including accuracy, sourcing, distinguishing opinion from fact, and providing context.
A June 2025 analysis published in the American Journal of Infection Control, based on Google News reports, found that less than 25% of all news stories cited clinical research.
Travel Vaccine Newsletters
Anyone can enroll in the free Vax-Before-Travel newsletter for the latest vaccination news. Recent studies have highlighted that when people are exposed to messages about vaccine options, about 49% report that the information is new, and 65% indicate that they are likely to discuss vaccines with their healthcare provider.
Contributing Expert Program
The VBT News Contributing Expert Program empowers vaccine providers to communicate with people digitally through news articles. This Trusted Travel Vaccination Network offers guidance and services to international travelers seeking unbiased information on preventing diseases while traveling.
Travel Vaccine Appointments
VBT enables travelers to request an appointment with a vaccine expert using this link.
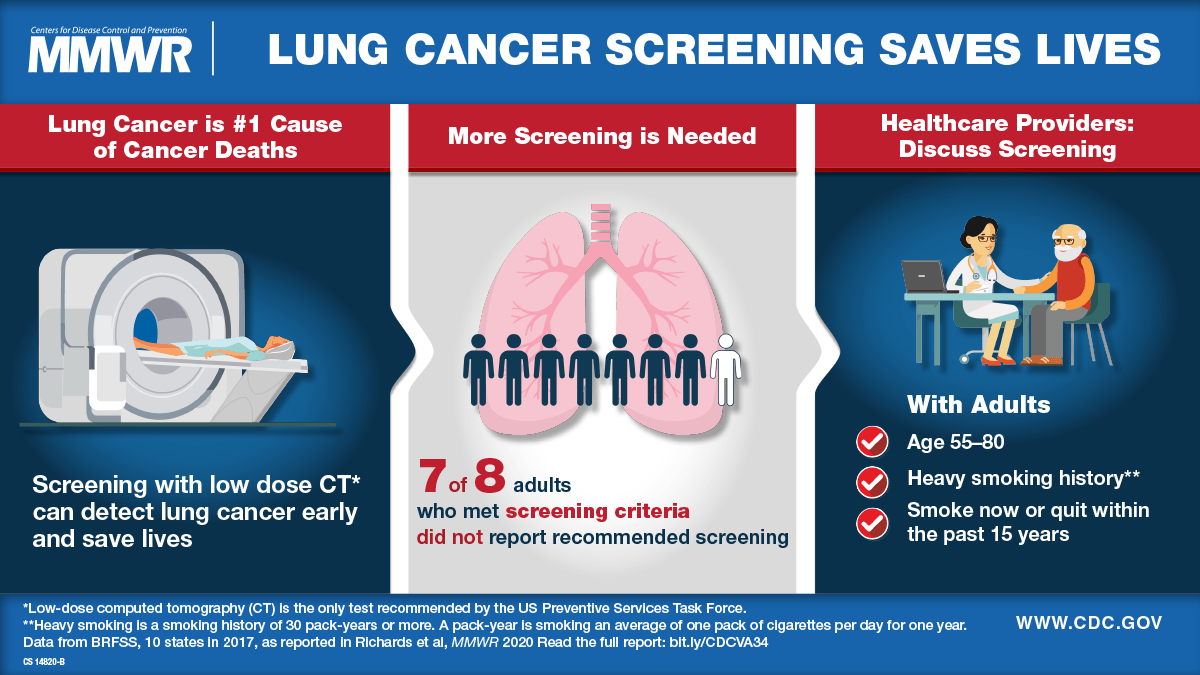
Travel Vaccination Overview
The U.S. Travel Association research reveals that about 1.4 billion passengers are expected to fly in 2025. Recent research suggests that tens of millions of people are not adequately vaccinated before traveling to countries with endemic diseases. This includes last-minute travelers who deferred approximately 18% of their protective vaccines due to insufficient time before departure.
According to Coherent Market Insights, The Global Vaccines Market is estimated to be valued at $81.91 billion in 2025 and is expected to reach $124 billion by 2032. The Medical Value Travel population, which travels to different regions or countries seeking healthcare services, was valued at $115 billion in 2022 and is expected to reach approximately $286 billion by 2030, representing a compound annual growth rate of 10.8%.
Travel Vaccination Timing
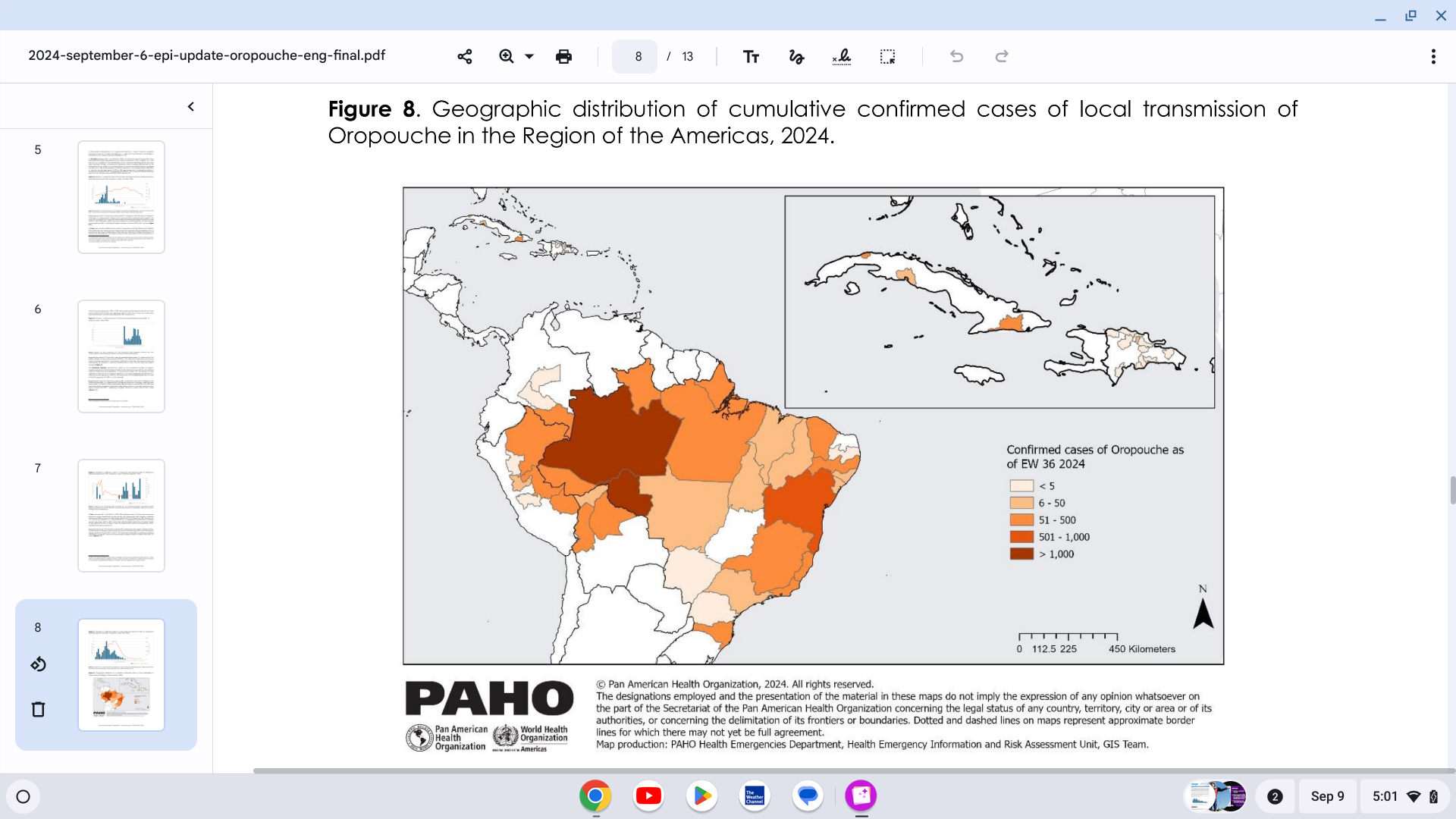
With over 15 million people traveling monthly, the U.S. CDC recommends that most travel vaccines be administered at least one month, if not earlier. The time between the vaccine's administration and the start of travel is significant for seniors. In Europe, over 4% of returning international travelers who recently displayed symptoms may be infected with a mosquito-transmitted disease, such as chikungunya, dengue, or Zika.
During pre-travel consultations, providers should consider potential interactions between vaccines and medications. A study by S. Steinlauf et al. identified potential drug interactions with travel-related medicines in 45% of travelers with chronic conditions; 3.5% of these interactions were potentially serious.
Policies
Various VBT policies are listed on this webpage.
Comments and Concerns
This link discusses VBT ownership and revenues. VBT is committed to providing greater transparency about how travel vaccine information is produced and distributed. We will respond to your request as soon as possible. Please email [email protected]. If you have any clinical questions, please get in touch with VBT's medical director, Dr. Robert Carlson, at [email protected].
Thank you,
Don Hackett, Founder & Publisher, VBT LLC