Search API
While the Republic of Indonesia has been battling tuberculosis cases for years, its focus in 2024 has turned to testing various vaccine candidates to curtail future outbreaks.
Despite having the second-highest number of tuberculosis cases, Indonesia has not participated in all previous vaccine studies.
According to an ANTARA News report on September 26, 2024, Indonesia is conducting clinical trials for three tuberculosis vaccines, including the M72/AS01E vaccine developed by the Bill & Melinda Gates Foundation.
Indonesia's Health Minister Budi Gunadi Sadikin commented, 'the need for more discussions and conferences to eliminate tuberculosis by 2030, including by taking bold and aggressive action, especially in the vaccine development process.'
As of October 15, 2024, over ten tuberculosis vaccine candidates were being researched in various countries, including India, the unfortunate leader in TB cases.
In the United States, Merck's TICE BCG vaccine is FDA-approved for the prevention of tuberculosis; however, it has various access limitations, including those applicable when visiting Indonesia.
The U.S. CDC recommends pre-trip vaccinations for chikungunya, Japanese encephalitis, measles, and polio, but not TB, when visiting Indonesia in 2024. These travel vaccines are typically available at clinics and pharmacies in the United States.
In Europe, the mosquito vector Aedes albopictus, established in many parts of Europe for years, transmits both chikungunya and dengue viruses.
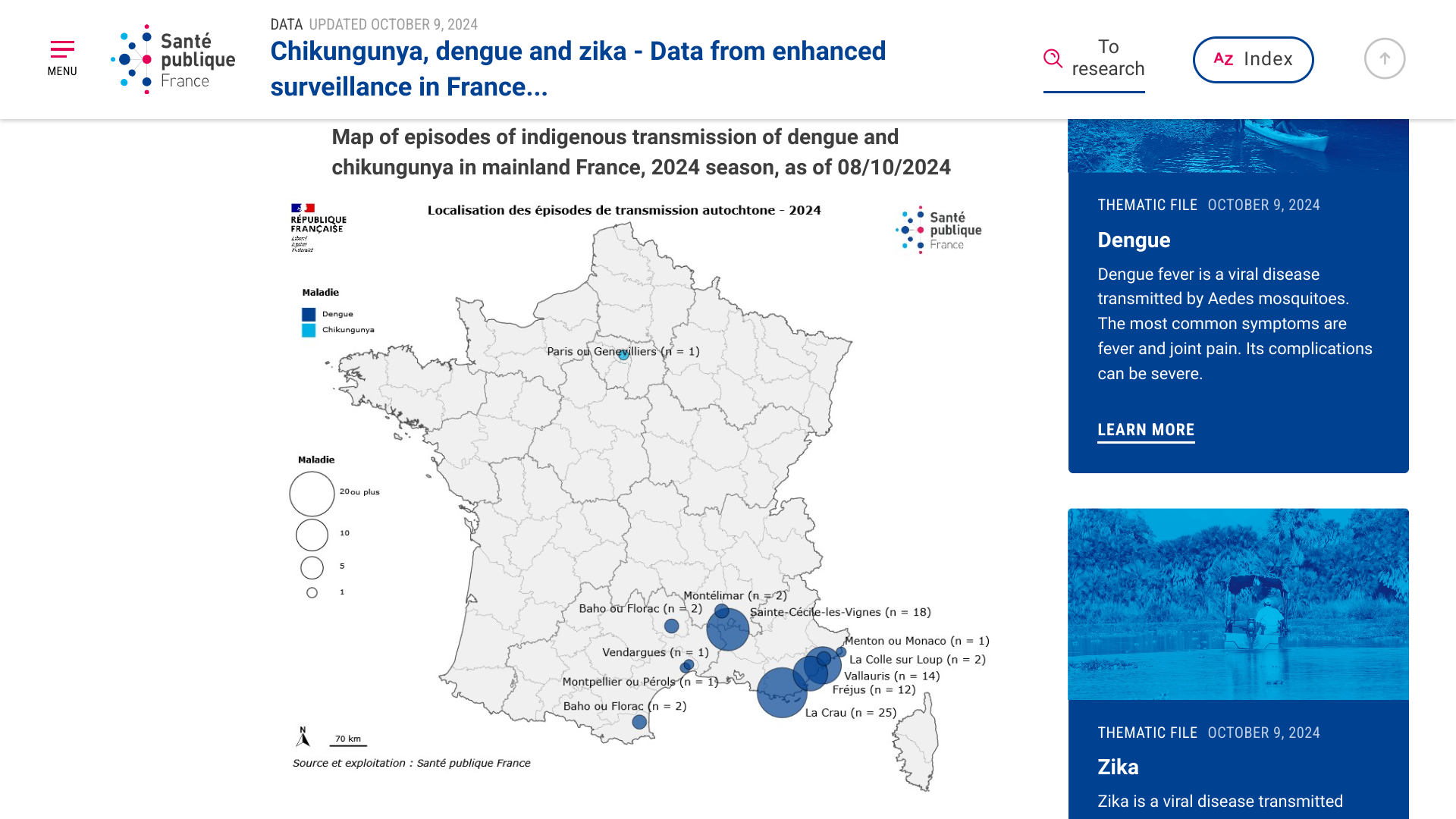
Recently, France's Public Health Ministry stated that detecting additional dengue and chikungunya cases in 2024 has prompted epidemiological and entomological investigations.
As reported on October 9, 2024, the majority of locally-acquired and travel-related cases have been detected in southern France.
From January to April 30, 2024, 2,271 travel-related dengue fever cases and six imported chikungunya cases were reported.
From May 2024 to October 8, 2024, 1,634 imported cases of dengue fever, including 1,468 in departments where the establishment of Aedes albopictus has been documented, and 16 imported cases of chikungunya.
Overall, France reported 78 locally acquired dengue cases in 2024. In 2023, France reported nine outbreaks of dengue involving 45 cases of autochthonous human dengue virus infections.
In the past, local dengue outbreaks have been reported in Italy, Spain, and Croatia.
As of October 15, 2024, the U.S. CDC says chikungunya and dengue are a year-round risk in many parts of the world, and international travelers should take action to prevent infections. The CDC has identified more than expected mosquito-transmitted cases among U.S. travelers returning from various countries.
RedHill Biopharma today announced that it had received a contract with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to advance the development of opaganib, a small-molecule treatment for Ebolavirus.
This novel, potentially broad-acting drug has shown mutation-resistant antiviral and anti-inflammatory activity, likely to counteract the vascular impacts of Ebola infection.
In a press release on October 14, 2024, the company stated that it is pursuing an animal-rule pathway for potential approval for this Ebola treatment candidate. This process is used when human clinical trials are not ethical or feasible.
Guy Goldberg, RedHill's Chief Business Officer, commented, "Currently, only Inmazeb™, a combination of three monoclonal antibodies, and Ebanga™, a single monoclonal antibody, are FDA-approved to treat Ebola infections. As such, there is an urgent need for additional effective and easy-to-distribute and administer therapies (during an outbreak)."
While there are approved Zaire Ebola vaccines and therapeutics available in 2024, previous outbreaks have highlighted significant logistical challenges that exist in managing Ebola outbreaks.
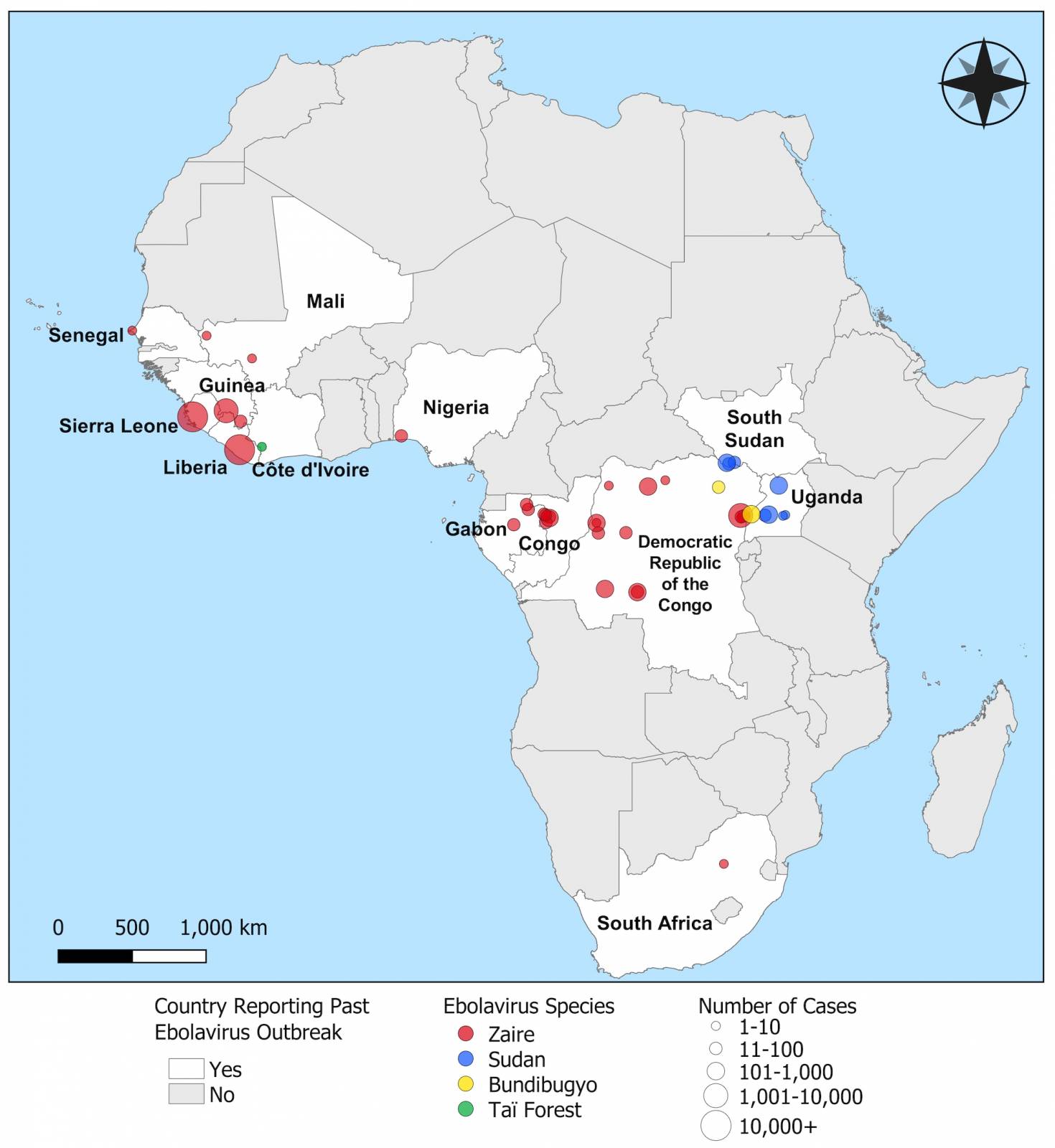
As of October 2024, more than 30 Ebola outbreaks have been reported in Africa. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River in Africa, and the virus's origins remain enigmatic in 2024.
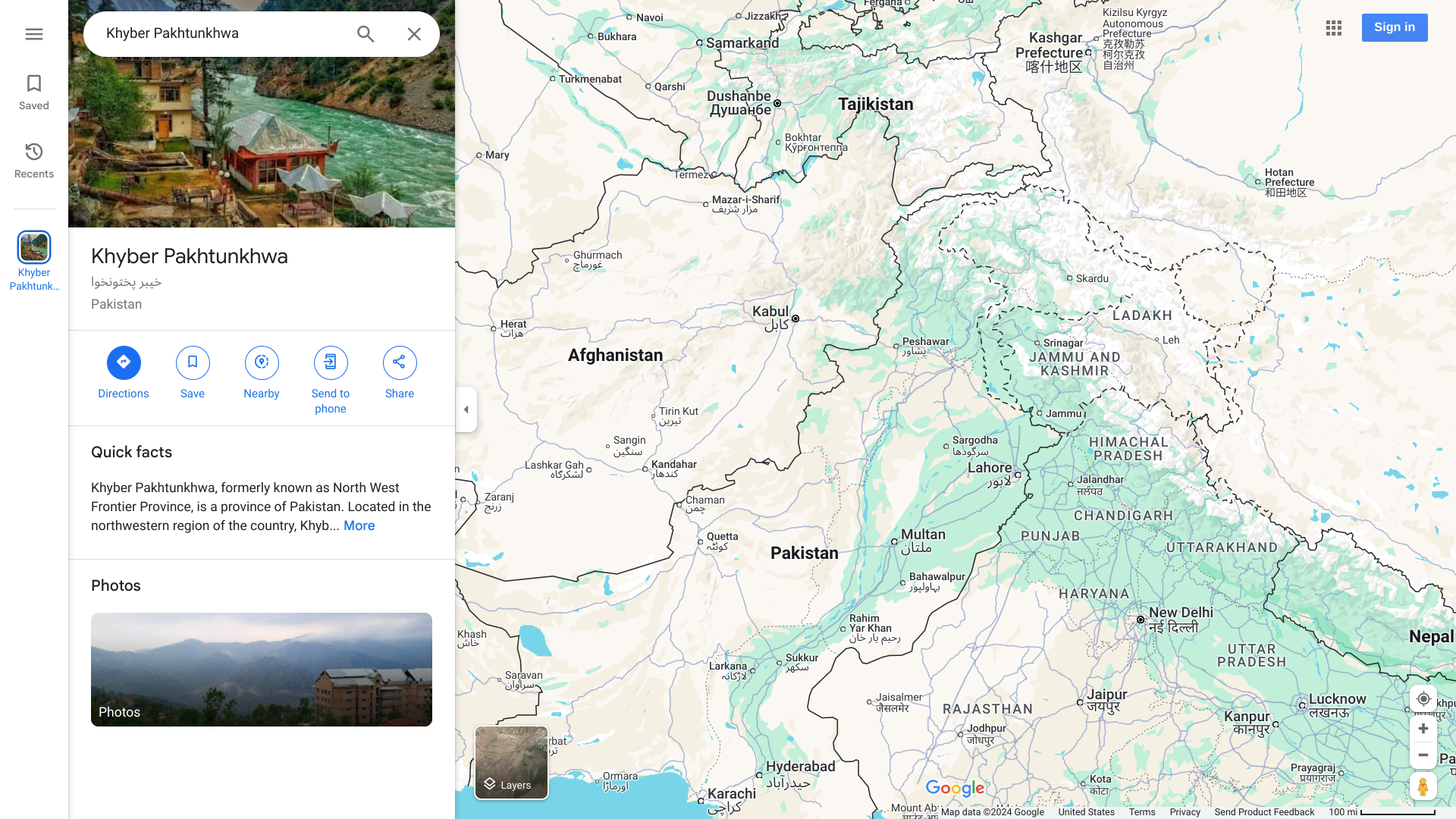
The Islamic Republic of Pakistan is one of two countries left in the world where poliovirus continues to threaten the health and well-being of its 250 million residents.
According to the weekly report published by the Global Polio Eradication Initiative (GPEI), Pakistan confirmed four new wild poliovirus type 1 (WPV1) cases. WPV1 is the only circulating wild poliovirus serotype.
The latest instances of paralysis were in Balochistan, Khyber Pakhtunkhwa, and Sindh provinces.
Furthermore, 50 WPV1-positive environmental samples were recently confirmed from Balochistan, Islamabad, Khyber Pakhtunkhwa, Punjab and Sindh.
As of October 14, 2024, Pakistan has reported 28 cases of WPV1 this year.
Since 1994, the Pakistan Polio Eradication Program has been fighting to end poliovirus infections. Through its efforts, case numbers in Pakistan have declined by up to 99% from the 20,000 cases reported in the early 1990s.
Unfortunately, the WHO reconfirmed in August 2024 that the spread of the poliovirus remained a Public Health Emergency of International Concern.
The U.S. CDC reported in September 2024 that routine immunization coverage with oral polio vaccines (OPV) and inactivated polio vaccine (IPV) in Pakistan has improved in recent years, as IPV protects against paralysis.
However, because it is an inactivated vaccine that does not replicate in the intestinal tract, as does OPV, it does not prevent the spread of poliovirus.
This could partly explain the relatively low number of WPV1 cases reported in the context of widespread WPV1 circulation as evidenced by environmental surveillance.
However, one-half of all WPV1 patients had never received OPV through routine immunization, indicating population immunity gaps. Whenever feasible, vaccination activities need to be synchronized with those of neighboring Afghanistan, says the CDC.
In Africa and Asia, the nOPV2 vaccine has been offered in 2024.
In the United States, the IPV has been offered since 2000, and booster doses are recommended for certain international travelers in 2024.
Next week, the U.S. CDC's Advisory Committee on Immunization Practices (ACIP) vaccine experts and staff will meet in Atlanta, Georgia, to review scientific data and vote on vaccine recommendations.
The agenda for the October 23-24, 2024, meeting includes presentations focused on chikungunya, influenza, pneumococcal, and RSV, but it is not limited to these diseases/vaccines.
Several ACIP votes are planned during the meeting. The vote language linked here is considered 'draft language.'
All ACIP votes and recommendations are not final until the CDC's Director approves them.
Members of the public interested in making an oral public comment are strongly encouraged to submit a request to the CDC no later than October 18, 2024.

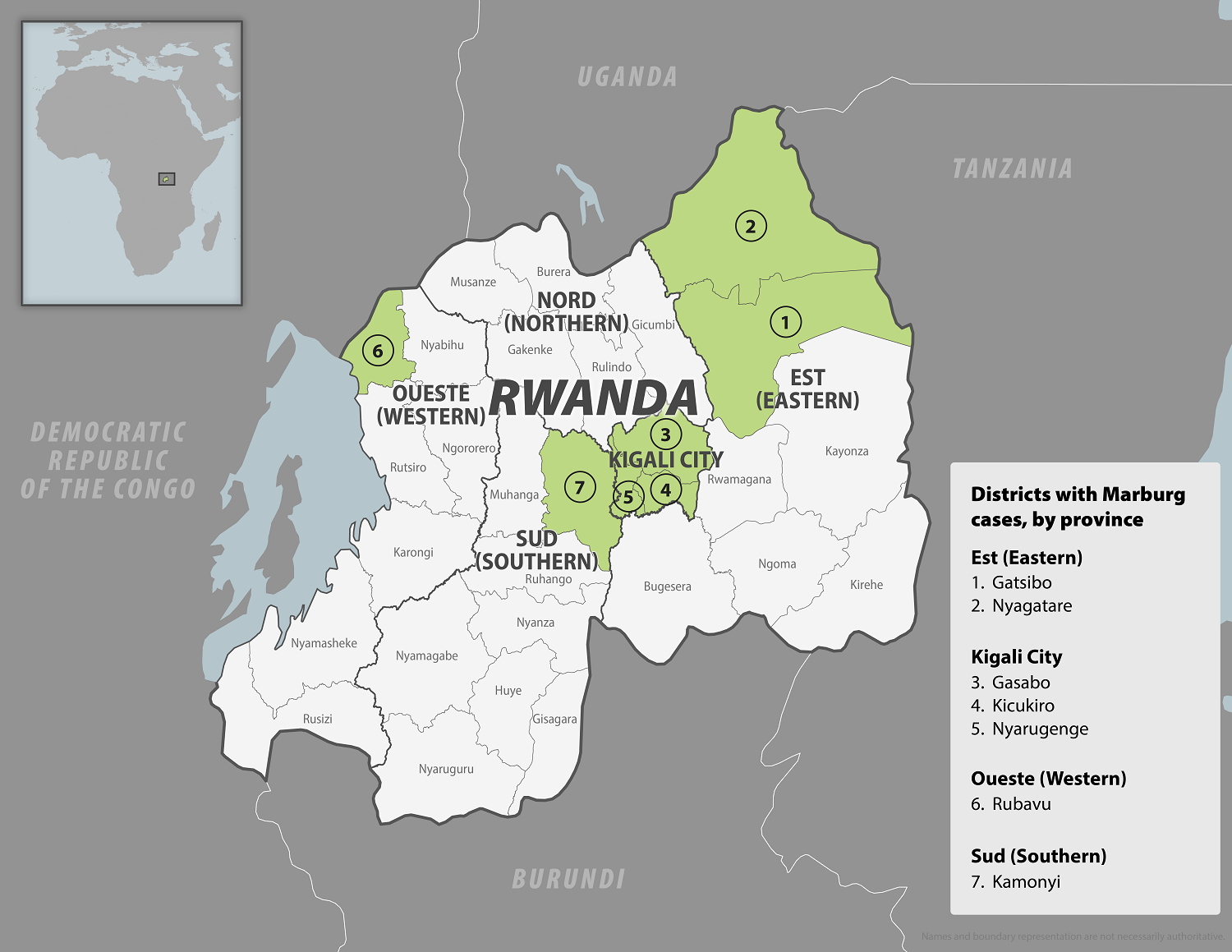
In early October 2024, the Republic of Rwanda began vaccinating frontline health workers in a Phase 2 rapid response open-label clinical trial to combat the reaction to the ongoing Marburg virus disease (MVD) outbreak, which has already claimed 14 lives.
Sabin Vaccine Institute’s single-dose Marburg vaccine candidate was selected to be administered in accordance with the clinical protocol reviewed and approved by Rwandan ethics and regulatory authorities. However, this is not a U.S. FDA-approved vaccine.
As of October 12, 2024, Sabin announced it had delivered approximately 1,700 investigational vaccine doses to Rwanda.
“In an outbreak, every moment counts, and our seamless collaboration with the Rwandan government was key to accelerating the process. On our side, we moved quickly by leveraging our experience with other outbreaks and having vaccine doses and supporting documents ready, thanks to a strong partnership with ReiThera,” says Sabin's CEO Amy Finan in a press release.
Sabin has extensive expertise in advancing vaccines for filoviruses, with two programs currently in Phase 2 clinical trials—one for Marburg and the other for Sudan ebolavirus.
The U.S. government has obligated $235 million to Sabin to advance vaccine research and development against Sudan ebolavirus and MVD.
As of October 13, 2024, other MVD vaccine candidates are conducting clinical research.
Previously, the U.S. CDC announced that people should reconsider nonessential travel to the Republic of Rwanda and that those who arrive in the U.S. may be screened for the virus at certain airports.