Search API
With avian influenza continuing to circulate the globe, various countries have invested in protecting people from a potential pandemic.
The UK Government today announced that CSL Seqirus UK Limited will manufacture more than five million doses of human H5 influenza vaccine.
This vaccine purchase, confirmed on December 3, 2024, was part of long-established plans to boost the UK's access to vaccines for a wider range of pathogens with pandemic potential.
The UK. Health Security Agency announced on September 26, 2023, that it signed an advance purchase agreement with CSL Seqirus to produce over 100 million influenza pandemic vaccines at the Liverpool facility if or when they are needed.
In a media release, Dr. Meera Chand, Emerging Infection Lead at the UK Health Security Agency, said, "It is important for us to be prepared against a range of different influenza viruses that may pose human health risks. Early access to vaccines saves lives."
"Adding vaccines to the interventions already available will help us prepare for a wider range of threats."
The influenza A(H5N1) virus has been causing a prolonged global outbreak, primarily in birds but including various mammals. If this virus were to start spreading among humans, of which there is no evidence at this stage, the human H5 influenza vaccine would be used.
Like the UK, the U.S. government has previously signed various agreements for avian pandemic vaccines.
The U.S. FDA authorized CSL Seqirus Inc. Audenz™ (Influenza A(H5N1) Monovalent Vaccine, Adjuvanted) cell-based vaccine on January 31, 2020. On November 14, 2013, the FDA licensed the I.D. Biomedical Corporation Influenza A (H5N1) Virus Monovalent Vaccine, Adjuvanted (STN#: 125419), to prevent H5N1 influenza disease.
These avian influenza vaccines are not commercially available.

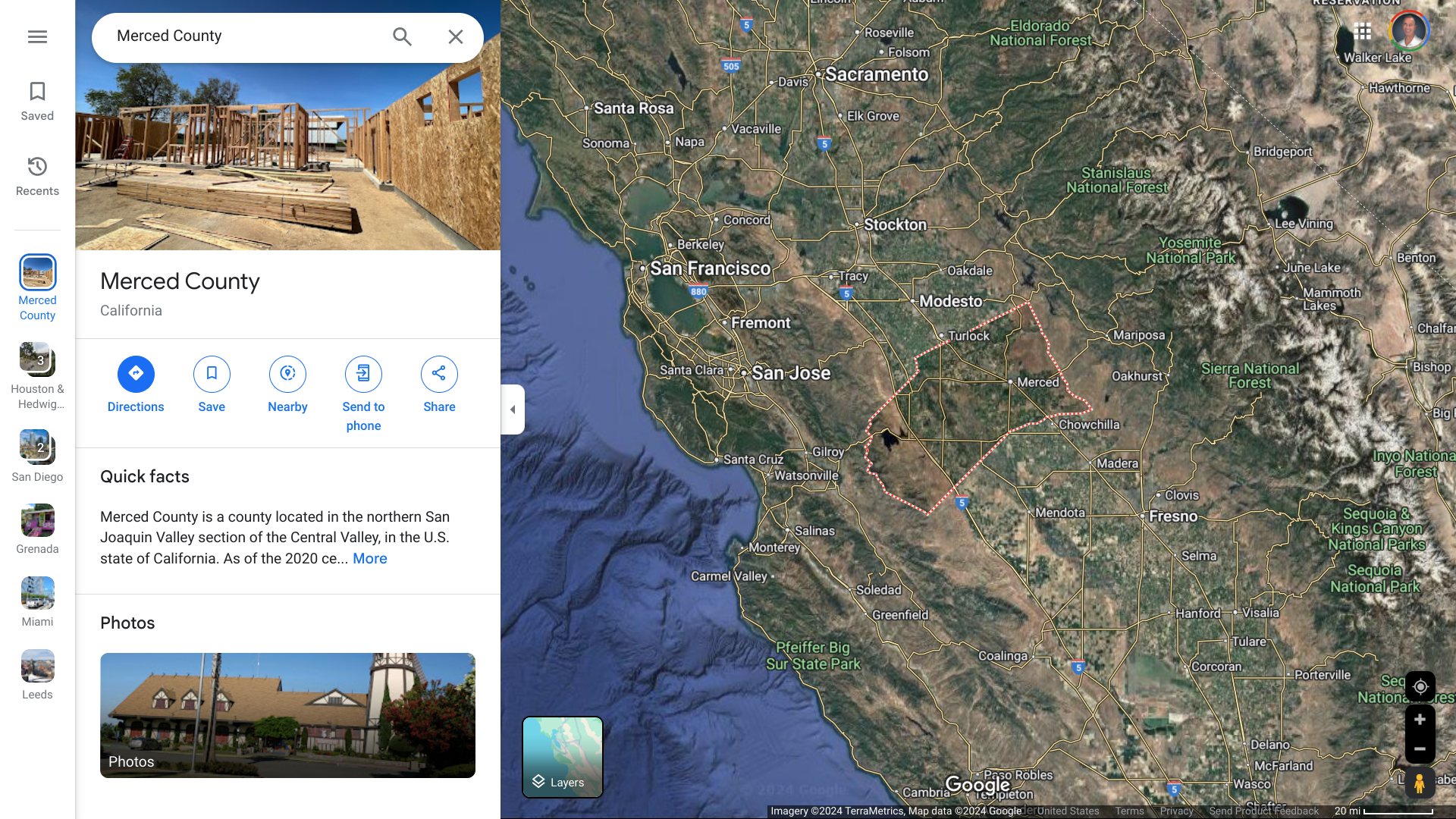
The Fresno County Department of Public Health (FCDPH) recently announced it is providing a media briefing on the rabies death involving a county resident who was suspected to have been bitten by a bat in Merced County, located east of San Jose.
The FCDPH stated in a media release that on November 26, 2024, the Merced County Department of Public Health notified individuals who may have been exposed and prepared medical providers in the community on response measures.
In September 2024, a Brantford-Brant, Ontario, resident was also confirmed with bat-bite transmitted rabies.
In the United States, bites from infected bats, not dogs, cause most rabies infections.
Rabies is a viral infection that causes brain and spinal cord inflammation. It is typically spread to humans through direct contact with saliva or mucous of an infected animal, such as through a bite or scratch.
According to the U.S. CDC, rabies vaccines have proven nearly 100% effective at preventing the disease after exposure. However, the vaccine must be started before rabies symptoms appear. Left untreated, rabies is almost always fatal.
Bavarian Nordic's RabAvert® vaccine is offered in the U.S.
After several years of steady increases in air travel, U.S. travelers set a new all-time record for passengers flown. Today, the Transportation Security Administration (TSA) released data surpassing previous Thanksgiving holiday volumes.
On Monday, 12/2/2024, 2,828,590 people passed through TSA security at an airport, exceeding 2,266,792 flyers in 2023.
And last Sunday, 3,087,393 people flew on a commercial airline, setting a new single-day record.
To meet this exceptional consumer demand, TSA adjusts processes and procedures to achieve the highest transportation security and customer satisfaction levels.
One area of service is TSA PreCheck®, where about 99% of passengers wait less than 10 minutes to pass through security. In 2024, more than 200 airports and 90+ airlines will provide TSA PreCheck®.
Additionally, the TSA uses canines as a critical component of its multilayered security strategy.
To celebrate this unique service, the TSA has released the 2025 Canine Calendar, an annual tradition honoring the agency’s more than 1,000 explosives detection canines working across the U.S.
The Calendar is now available for immediate download.

After receiving delivery of about 1 million R21/Matrix-M™ malaria vaccines last month, the Nigerian federal government has officially commenced its malaria vaccination program in Kebbi and Bayelsa states.
The R21 vaccine has also been integrated into the Federal Republic of Nigeria's National Immunization Schedule.
On December 2, 2024, local media reported that Prof. Muhammad Ali Pate, the Coordinating Minister for Health & Social Welfare, commented, "Malaria continues to exert an unacceptable toll on Nigeria, with 27% of global malaria cases and 31% of global malaria deaths, our country bears the heaviest burden of this disease. In 2022, over 180,000 Nigerian children under the age of five lost their lives to malaria- a tragedy we have the tools to prevent”.
The Serum Institute of India malaria vaccine was released in 2023. Significant shipments to Africa began in May 2024, and the company anticipates distributing over 50 million doses annually.
Time USA recently included R21 in its list of the 'Best Inventions of 2024.'
The R21 vaccine is unavailable in the United States.
The U.S. CDC says that when visiting Nigeria in 2024, visitors should be aware of current health issues, such as measles, diphtheria, polio, and yellow fever.
Valneva SE today reported positive antibody persistence data three years after vaccination with a single dose of its chikungunya vaccine IXCHIQ®.
Among the healthy adults still enrolled in a clinical trial, 96% maintained neutralizing antibody titers.
Announced on December 3, 2024, the results align with Valneva's expectations for the only U.S. FDA-approved chikungunya vaccine, confirming a strong and long-lasting antibody persistence across all age groups investigated.
IXCHIQ's three-year persistence data also align with positive twelve-month and two-year persistence data the Company reported in December 2022 and 2023, respectively.
These data are positive news for the Region of the Americas, where 412,094 mosquito-transmitted chikungunya cases and 204 related fatalities have been reported this year.
In the United States, the Centers for Disease Control and Prevention reported 173 travel-related chikungunya cases in Territories and non-U.S. residents in 2024, led by Massachusetts (20) and Texas (20).
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "We are extremely pleased about these three-year data, which further highlight IXCHIQ®'s differentiated product profile and ability to induce a robust, long-lasting antibody response in both younger and older adults with a single vaccination."
"Whether you're a traveler or live in an endemic region, the potential for long-term protection against a mosquito-borne disease with a single dose is crucial, particularly in low- and middle-income countries where vaccine access is often limited."
IXCHIQ® is the world's first and only licensed chikungunya vaccine approved in the U.S., Europe, and Canada to address this significant unmet medical need.
The Company expects a marketing authorization in Brazil, a chikungunya hot spot, before the end of 2024.
In the U.S., IXCHIQ is available at travel clinics and pharmacies, such as Passport Health in Tampa, Florida.

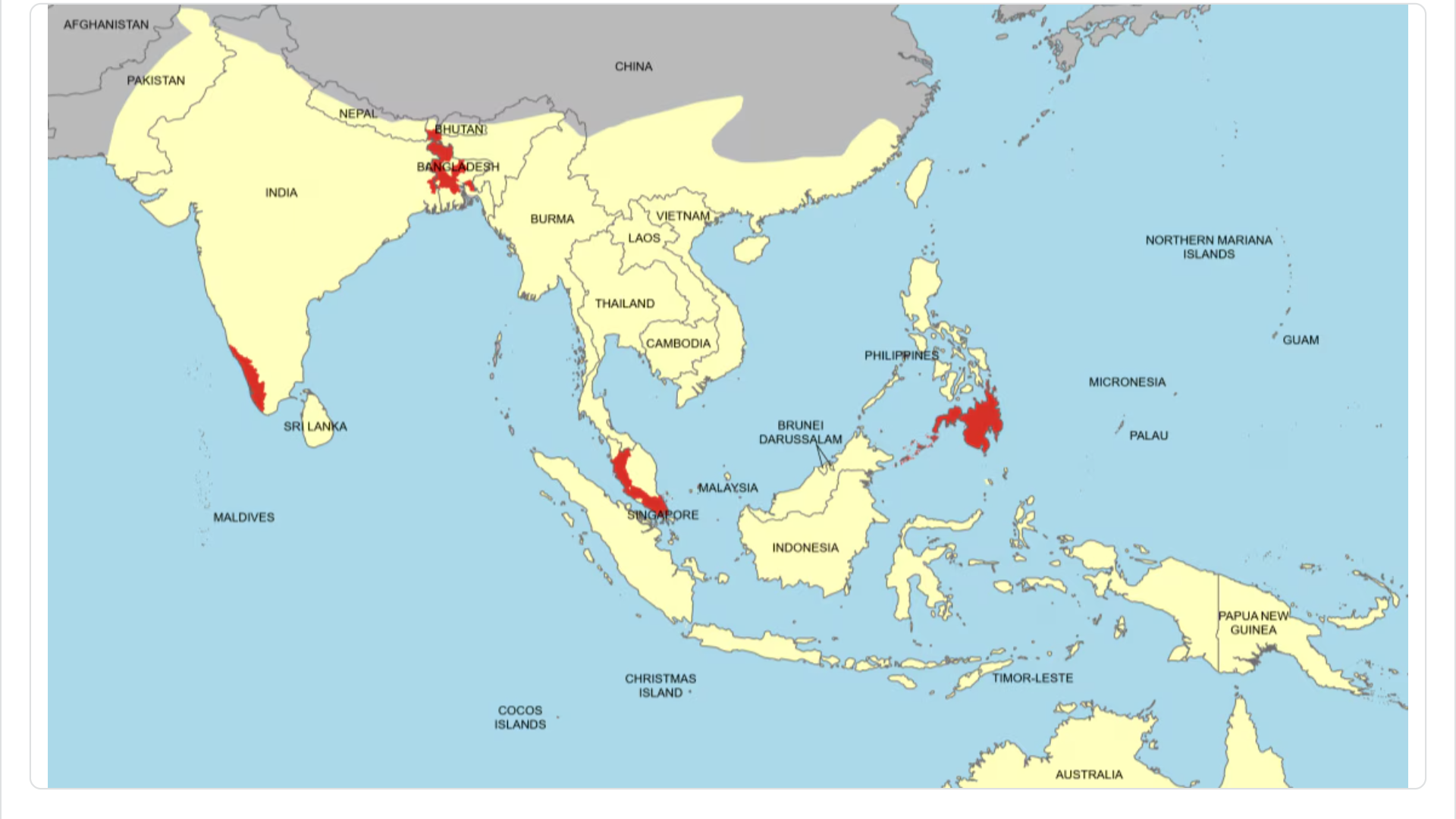
Since 2016, the Zika virus (ZIKV) has been reported in India's 16 different states/union territories. According to local media, the ongoing Zika outbreak in the state of Maharashtra, India, has significantly expanded this year.
As of November 29, 2024, Maharashtra's Zika outbreak has reached 140 cases, five related fatalities, and 63 pregnant women. The majority of these cases are located in Pune.
While most Zika cases result from a bite from an infected mosquito, the virus is transmitted through sexual contact. Scientists have reported that ZIKV RNA is detectable in the semen of infected men for months.
Zika virus can be passed from a pregnant person to their fetus and can cause microcephaly, a medical condition involving a smaller-than-normal head.
With a population exceeding 7 million and over 1 million international visitors, the U.S. Centers for Disease Control and Prevention (CDC) have maintained a Level 2—Practice Enhanced Precautions, Travel Health Advisory for this state in India since August 22, 2024.
The CDC recommends that pregnant women avoid travel to Maharashtra.
If travel is unavoidable, follow the Zika prevention recommendations strictly. And if you are planning pregnancy, you should delay pregnancy following travel based on the timeframes to prevent sexual transmission.
Travelers to Maharashtra should seek medical care immediately if they develop fever, rash, headache, joint or muscle pain, or red eyes during or after travel.
As of December 2024, the CDC reported 28 non-congenital Zika cases in the U.S. In Puerto Rico, the Department of Health says 16 Zika cases have been confirmed in 2024.
As of December 2, 2024, no approved Zika vaccine is available. However, Valneva SE's VLA1601 is a second-generation purified, inactivated, whole Zika vaccine candidate actively conducting clinical trials.
The Lancet Infectious Disease recently published a Correspondence focused on Nipah virus research priorities.
Nipah was first discovered in 1999 following an outbreak in pigs and people in Malaysia and Singapore. Since then, outbreaks have occurred nearly every year in many parts of Asia, often in Bangladesh (2023) and India (2024).
According to the U.S. CDC, around 40%–70% of people infected with Nipah die.
On November 18, 2024, these authors wrote, 'WHO's roadmap for Nipah virus research priorities (2024–29) outlines ambitious milestones for advancing diagnostics, therapeutics, and vaccines.
Although the roadmap signifies progress against the Nipah virus, it also exposes inequities in global health governance, raising concerns about who sets research agendas and for whose benefit.
We sincerely commend the distinguished experts involved in the roadmap development process for their exceptional leadership, expertise in henipaviruses, including Nipah and Hendra viruses, and representation of affected regions.
However, the low representation from the most affected countries highlights gaps in the priority-setting process. Of the 26 authors, only 3 (11%) were from the two countries—Bangladesh and India—that reported all Nipah virus cases in the last two decades, and other affected countries, including Malaysia and the Philippines, were not represented.
This exclusion contrasts with the roadmap's objective of addressing needs in regions most vulnerable to outbreaks.
To make meaningful progress, the global health community must adopt an inclusive approach to research prioritization and co-developing roadmaps with stakeholders from affected regions, including clinicians, public health practitioners, and community leaders. This approach will ensure that priorities are scientifically robust, implementable, and culturally relevant.
The unedited correspondence is found at this link.
In August 2024, Phylex Biosciences announced its new mRNA nanoparticle vaccine against the Nipah virus. The vaccine achieved positive results in an immunogenicity study conducted in collaboration with scientists from the U.S. CDC.
As of December 2, 2024, the CDC has not approved a vaccine against the Nipah virus.