Search API
The World Health Organization (WHO) recently informed its Member States of a suspected Marburg Virus Disease (MVD) outbreak in the Kagera region of the United Republic of Tanzania.
On January 13, 2025, the WHO wrote that the risk of this suspected MVD outbreak is assessed as high at the national level due to several concerning factors.
The regional risk is also considered high due to the Kagera region's strategic location as a transit hub, with significant cross-border movement of the population to Rwanda, Uganda, Burundi, and the Democratic Republic of the Congo.
As of early January 2025, nine suspected cases were reported, including eight deaths (case fatality ratio of 89%) across two districts – Biharamulo and Muleba.
Close contacts, including healthcare workers, are reported to have been identified and under follow-up in both districts. Human-to-human transmission of Marburg virus is primarily associated with direct contact with the blood and/or other bodily fluids of infected people.
The Bukoba district in the Kagera region experienced its first MVD outbreak in March 2023, and zoonotic reservoirs, such as fruit bats, remain endemic to the area. The outbreak in 2023 lasted for nearly two months, with nine cases and six deaths.
Since 1967, MVD outbreaks have been confirmed in various countries.
The WHO advises against travel and trade restrictions with Tanzania based on the current risk assessment.
As of January 15, 2025, the U.S. CDC issued a Level 1 - Practice Usual Precautions, Travel Health Advisory for visiting Tanzania. Previously, the CDC included Tanzania in its current global polio Travel Health Advisory.
Furthermore, no U.S. FDA-approved Marburg vaccines exist, but candidates are conducting clinical trials.

Today, Micron Biomedical announced that the U.S. Department of Health and Human Services (HHS) awarded the company $2 million to continue its work on broadly protecting avian and seasonal flu vaccines.
The company won the Biomedical Advanced Research and Development Authority (BARDA) Patch Forward competition. The $2 million award will advance Micron’s work to co-develop needle-free versions of broadly protecting influenza vaccines with Zipcode Bio, a biotechnology company focusing on next-generation RNA medicines.
“Making all vaccines, including influenza vaccines, more broadly protecting and more accessible has the potential to save lives globally, and we are thrilled to be recognized by the HHS, BARDA, as one of the most important vaccine innovations rethinking how vaccines will be administered,” said Steven Damon, CEO of Micron Biomedical, in a press release on January 14, 2025.
“We are thrilled to continue our collaboration with Zipcode Bio and accelerate efforts to make the dream of needle-free, broadly protecting influenza vaccines a reality.”
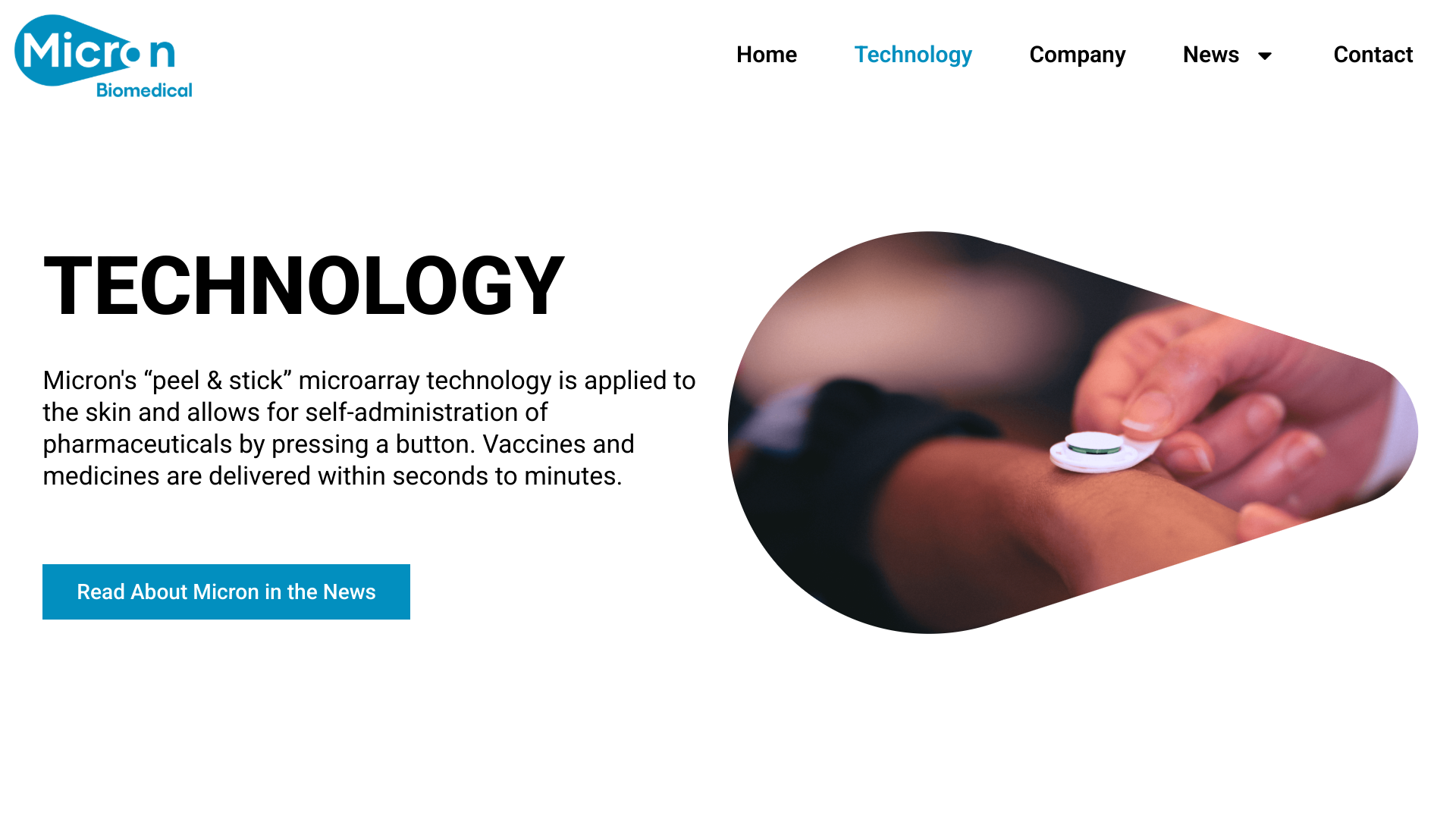
Micron’s technology uses a dissolvable microarray “button” applied to the skin. The button painlessly delivers a vaccine or therapeutic directly into the uppermost dermal layers when pushed. The button can be self-administered or administered by a caregiver or parent.
Micron previously announced that it had received a $7.5 million grant from the Bill & Melinda Gates Foundation, for a total of $43 million in support from the organization.
Over the past decade, the World Health Organization (WHO) and the National Academy of Sciences have written, A future influenza pandemic is inevitable.... are we ready?
From January 2003 to November 2023, the WHO reported 246 cases of human infection with the avian influenza A(H5N1) virus from four WHO Western Pacific Region countries. Of these, 138 were fatal, resulting in a case fatality rate (CFR) of 56%.
According to the U.S. Centers for Disease Control and Prevention and the Department of Agriculture, mammalian infections with the highly pathogenic avian influenza virus will be a global concern in 2025.
As of early 2025, the U.S. government has invested in developing avian influenza vaccine candidates.
Emergent BioSolutions Inc. today announced that the Biomedical Advanced Research and Development Authority (BARDA) executed a contract modification for the second option period, valued at approximately $16.7 million.
This option is part of Emergent's existing 10-year contract with BARDA for the advanced development and procurement of Ebanga™, which has a maximum value of $704 million.
This modification will validate the drug product process and analytical testing and ensure long-term stability for Ebanga, which is indicated for treating infection caused by the Zaire Ebola virus.
Ebanga (ansuvimab-zykl) is a Zaire ebolavirus glycoprotein-directed human monoclonal antibody indicated for treating infection caused by Zaire ebolavirus in adult and pediatric patients.
As of 2025, the U.S. Department of Homeland Security has determined that Ebolavirus disease (EVD) threatens national health security. To augment the government's response capability, BARDA is pursuing the advanced development, licensure, and procurement of therapeutics that can be deployed in EVD outbreaks.
"We are delighted our continued collaboration with BARDA is advancing Ebanga development toward supplying treatment and ensuring communities are prepared against Ebola (outbreaks)," said Simon Lowry, M.D., chief medical officer, head of research and development, Emergent, in a press release on January 13, 2025.
"Ebola is a devastating infectious illness with limited treatment options."
Ebanga is not a preventive vaccine.
As of early 2025, Merck's U.S. FDA-approved Ervebo® (rVSV-ZEBOV) vaccine was licensed in the U.S., the U.K., the European Union, Canada, and various countries. Recently, Sierra Leone became the first country in Africa to launch a preventive Ebola vaccination campaign targeting health workers.
Ervebo is not commercially available in the U.S.
Orthoebolavirus zairense (EVD) is severe and often fatal, with case fatality rates ranging from 25% to 90%, and is transmitted via bodily fluids, zoonotic transmission, or contact with contaminated surfaces.
According to the World Health Organization, more than 30 EVD outbreaks have been reported. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River.
As of 2025, no active U.S. CDC Travel Health Notice is focused on Ebola outbreaks in Africa.

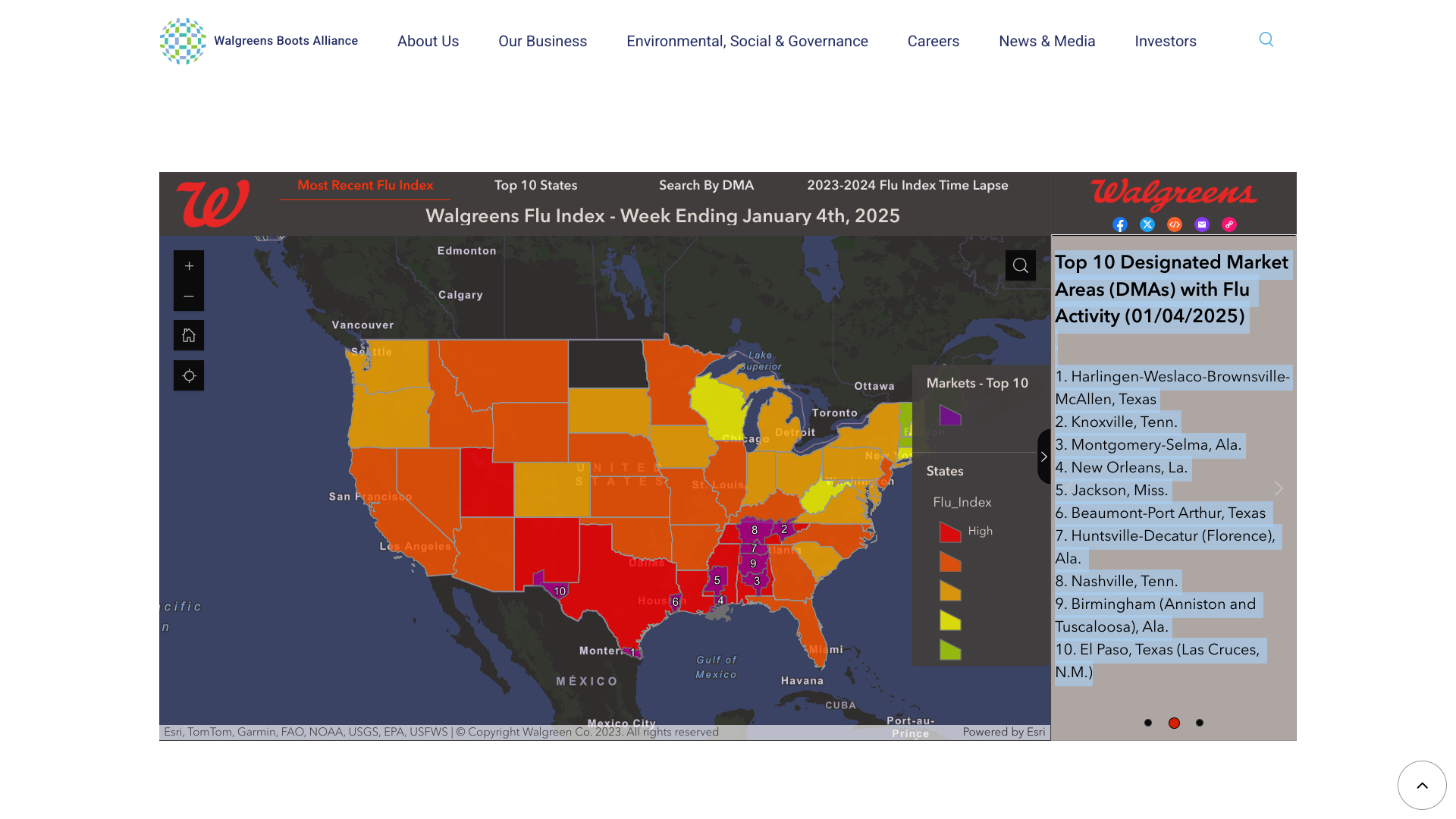
The Walgreens Flu Index® recently highlighted the top 10 areas in the United States with influenza activity.
As of January 4, 2025, the cities were primarily located in the south-central U.S.
- Harlingen-Weslaco-Brownsville-McAllen, Texas,
- Knoxville, Tenn.,
- Montgomery-Selma, Ala.
- New Orleans, La.,
- Jackson, Miss.,
- Beaumont-Port Arthur, Texas,
- Huntsville-Decatur (Florence), Ala.,
- Nashville, Tenn.,
- Birmingham (Anniston and Tuscaloosa), Ala.
- El Paso, Texas (Las Cruces).
The Walgreens Flu Index provides state—and market-specific information regarding flu activity. It is compiled using retail prescription data for antiviral medications used to treat influenza at Walgreens's thousands of pharmacies nationwide, including Puerto Rico.
The Flu Index is not intended to illustrate flu activity levels or severity, but it complements the weekly data posted by the U.S. CDC. It is an interactive tool that allows users to search by market or state to see where their geographic area ranks for flu activity in any given week.
In 2024, over 92 million flu shots were distributed in the U.S. As of January 13, 2025, various vaccine types remain available at most community pharmacies. They are recommended for most children and adults, and booster doses are often suggested for certain people.
According to the World Health Organization (WHO), the clade Ib monkeypox virus (MPXV) outbreak began in September 2023 and continues predominantly in the Democratic Republic of the Congo, Burundi, and Uganda, with travel-related cases identified in other countries.
In Africa, from January 2024 to January 5, 2025, 14,700 confirmed mpox cases, including 66 deaths (CFR – 0.4%), have been reported by 20 countries. And continues to meet the WHO criteria for a public health emergency of international concern.
As of January 14, 2025, the ECDC reported eleven individuals with MPXV clade I in the EU/EEA since August 2024.
One case was reported by Sweden in August 2024, seven by Germany (one in October, five in December 2024, and one in January 2025), two cases by Belgium in December 2024, and one case by France in January 2025
The WHO says two virus types cause mpox, clade I and II. Both types spread the same way and can be prevented using the same methods, including vaccination.
Most mpox outbreaks in other areas are due to clade IIb MPXV, a continuation of the multi-country outbreak that began in May 2022.
In the United States, the CDC assessed on January 10, 2025, the overall risk to the population(s) posed by the clade I mpox outbreak as low. And clade II mpox is still circulating at low levels.
Various mpox vaccines continue to be available in impacted countries.
Note: Updated on Jan. 14, 2025, to include ECDC data.

Curevo Vaccine today announced positive updated immunogenicity and safety data from its Phase 2 trial of amezosvatein (CRV-101) head-to-head versus Shingrix® in participants 50 years of age and older.
“The Day 421 Phase 2 data continue to support our view that amezosvatein has a comparable effect on the human immune system as Shingrix,” said Dr. Guy De La Rosa, Curevo’s Chief Medical Officer, in a press release on January 12, 2025.
CRV-101 is a non-mRNA adjuvanted subunit vaccine. Similar to Shingrix, amezosvatein uses a subunit protein antigen called glycoprotein ‘E’ (gE). Targeting the gE antigen is proven to elicit a long-term, protective immune response to prevent shingles.
Also, like Shingrix, amezosvatein uses an adjuvant targeting the TLR4 pathway to boost the immune response to the gE antigen.
The SLA-SE adjuvant formulation was developed at the Access to Advanced Health Institute. Amezosvatein was licensed from the Mogam Institute for Biomedical Research, a research institute funded by South Korea’s GC Biopharma.
“Amezosvatein’s non-inferior immunogenicity data and comparable herpes zoster case data, combined with amezosvatein’s improved tolerability versus Shingrix in this Phase 2 trial we reported this time last year, provide us with great confidence and excitement to continue development of this vaccine.”
“This is in a market expected to be worth over $5 billion in 2025,” noted George Simeon, Curevo’s Chief Executive Officer.
Currently, the U.S. CDC recommends two doses of the recombinant zoster vaccine (RZV, Shingrix) to prevent shingles and related complications in adults over> 50. And the CDC recommends two doses of RZV for adults who are or will be immunodeficient or immunosuppressed.
In 2025, Shingrix is offered at various pharmacies.

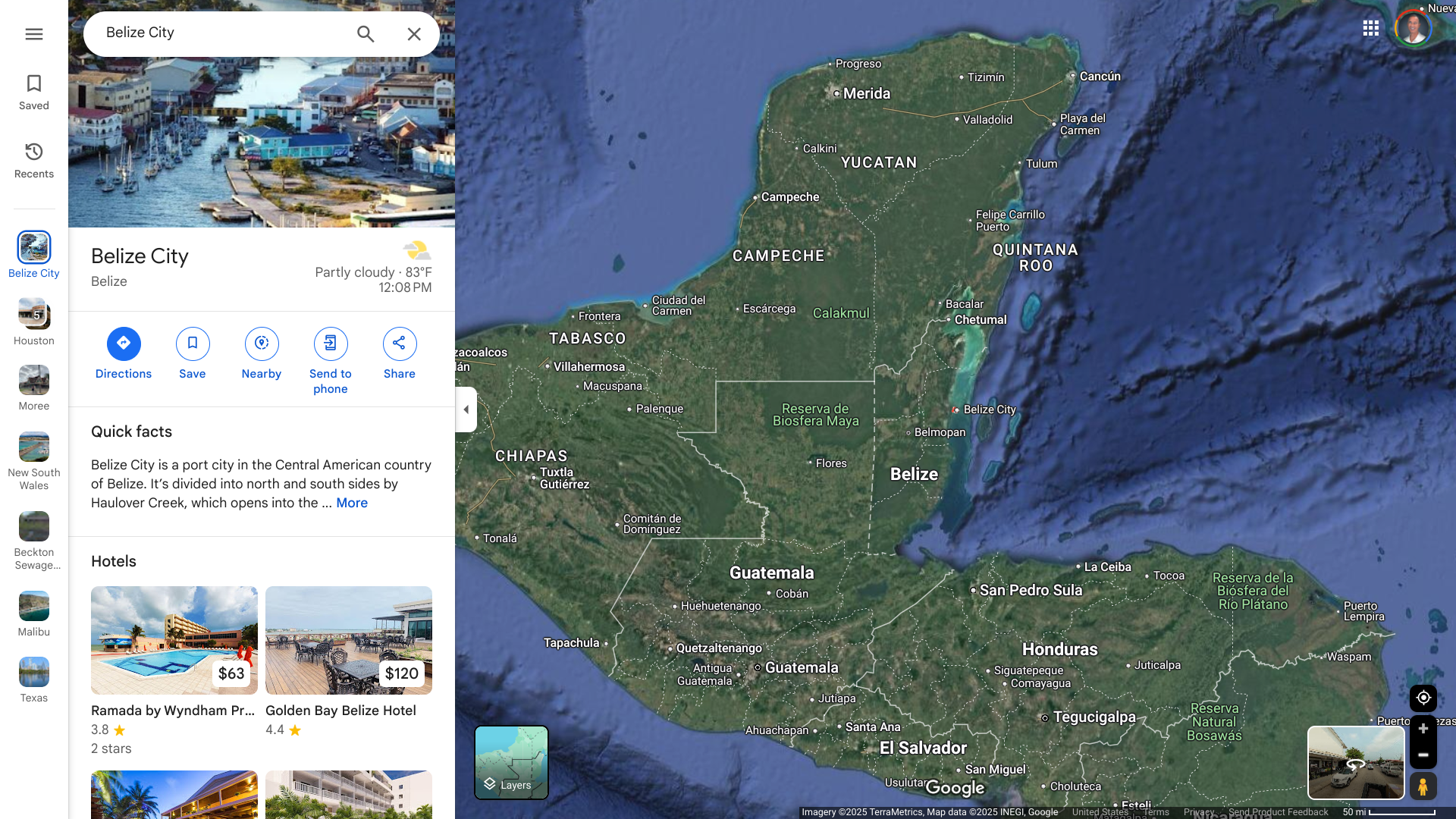
When the U.S. Department of State recently updated its Level 2: Exercise Increased Caution for the Central American country of Belize, it also highlighted various risks.
On December 30, 2024, the State Department advised travelers to exercise caution when exploring the south side of Belize City due to civil unrest. This area (south of Haulover Creek Canal and continuing south to Fabers Road) does not overlap with the typical tourism areas.
When visiting Belize in 2025, enroll in the Smart Traveler Enrollment Program to receive digital alerts and make locating you in an emergency easier. You can also see the local U.S. Embassy.
Belize is located on the Caribbean's east coast, just south of the Mexican state of Quintana Roo. In 2024, about 560,000 people visited the country.
From a health perspective, the U.S. CDC and the Pan American Health Organization (PAHO) reported that mosquito-transmitted Chikungunya, Dengue, and Zika viruses continued to impact Belize in 2024.
At the end of 2024, the PAHO confirmed 36 Chikungunya, 1,148 Dengue, and 31 Zika cases.
In 2024, the CDC stated that there had been evidence of Chikungunya virus transmission in Belize within the last five years. To prevent this disease, the new Chikungunya vaccine is an option for certain travelers.
As of January 12, 2025, the U.S. FDA-approved IXCHIQ® single-dose Chikungunya vaccine is commercially available at travel clinics and pharmacies.
While Chikungunya and Dengue virus outbreaks reached records in the Region of the Americas in 2024, a little-known virus with similar symptoms has been spreading, causing complications in diagnosis.
Oropouche virus, primarily transmitted through bites from infected midges, has expanded its range over the past year, reaching Central America and the Caribbean.
Research published in The Lancet Infectious Diseases in December 2024 estimates that up to 5 million people in the Americas are at risk of exposure to the virus.
To confuse a diagnosis, Oropouche infections can appear clinically similar to Chikungunya, Dengue, Malaria, and Zika. A reverse transcriptase–polymerase chain reaction test is only administered after a negative dengue result.
Still, once confirmed, no treatment or preventive vaccine for Oropouche is available as of January 2025.
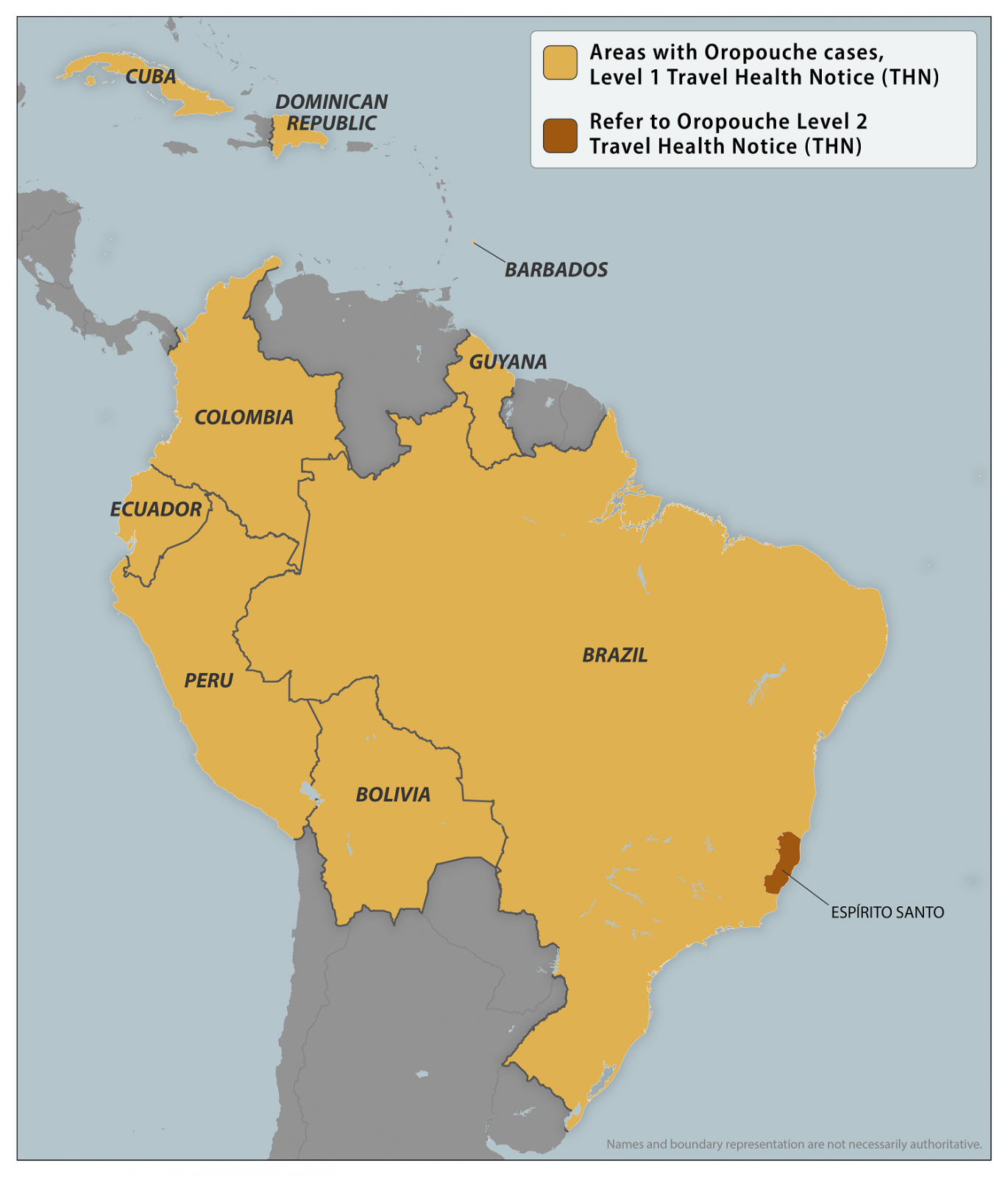
To alert international travelers to this health risk, the U.S. CDC updated its Level 1 - Practice Usual Precautions, Travel Advisory on December 18, 2024. The CDC lists nine countries that have reported Oropouche cases.
And in Brazil, the CDC issued a Level 2 Advisory for Espírito Santo.
In Florida, about 103 international travelers have been diagnosed with this infection.
Symptoms of Oropouche infection include headache, fever, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light. Severe cases may result in neuroinvasive diseases such as meningitis.
Furthermore, the CDC says the Oropouche virus has been found in semen and may spread through sex.