Search API
While the South Indian Ocean chikungunya outbreak has been focused on France's Réunion Island, the Republic of Mauritius has also reported imported and local cases in 2025.
According to the Mauritius health services, most mosquito-transmitted chikungunya cases were imported from Asia and Africa.
On March 17, 2025, Mauritius, located east of Africa and Madagascar, reported the first local case in the country since 2009.
Then, on April 8, 2025, the World Health Organization (WHO) reported 17 local chikungunya cases in Mauritius.
The WHO says virus transmission persists in populations with low prior exposure, making vaccines essential to a comprehensive chikungunya outbreak response.
As of April 10, 2025, the U.S. CDC has not issued a Travel Health Notice regarding this chikungunya outbreak.
For travelers departing from the United States, the United Kingdom, or Europe, effective chikungunya vaccines, such as IXCHIQ®, have been approved by government agencies and are commercially available at most travel clinics and pharmacies.
While most malaria cases are detected in Africa, on the other side of the world, malaria outbreaks continue to be detected in South Korea.
According to a post on X, the South Korean government has recently confirmed locally acquired malaria cases and is taking immediate action.
On April 9, 2025, the Ministry of National Defense wrote, 'Recent outbreak of malaria patients among soldiers discharged from military service in border areas such as Paju, Gyeonggi Province, and Cheorwon, Gangwon Province.'
The Ministry of National Defense and the Korea Disease Control and Prevention Agency are working to achieve a 'Malaria-Free Republic of Korea' to eradicate malaria domestically by 2030.
The Joint establishment of the "Second Malaria Eradication Action Plan (2024-2028) includes:
- Free malaria diagnostic tests and treatment prescriptions for one year after discharge at 14 military hospitals nationwide.
- Free diagnostic testing is available two years after discharge at public health centers nationwide.
In 2024, the Agency issued malaria warnings for areas, including Seoul's Yangcheon and Gangseo districts. In 2023, over 719 cases of the mosquito-borne disease were confirmed.
According to the U.S. CDC, travelers going to areas of South Korea should take prescription medicine to prevent malaria. Depending on the medicine you take, you will need to start taking this medicine multiple days before your trip, as well as during and after your trip.
As of April 10, 2025, malaria vaccines are offered in Africa, but not South Korea or the United States.

The Switzerland Federal Office of Public Health today reported its first case of Clade Ib mpox in an individual who had returned from Africa.
On April 8, 2025, the Swiss government stated that this person was in isolation and that there was no risk of infection to others.
The treatment of mpox consists primarily of treating its symptoms. In particularly severe cases, an antiviral therapy may also be conducted. The appropriate medicine is available in Switzerland in 2025.
Since September 2023, the World Health Organization has observed increased infections, especially of a new mpox variant designated Clade Ib, in Several African countries, including the Democratic Republic of the Congo.
The other well-known Clade II began infecting people worldwide in May 2022.
A preventive vaccination (Jynneos®) is assumed to be effective against Clade I infection. The vaccine is safe and highly effective in preventing severe mpox infections.
Jynneos is also available in numerous countries, such as the United States.

The South Korean Ministry of Food and Drug Safety recently approved BARYTHRAX, an anthrax vaccine jointly developed by GC Biopharma and the Korea Disease Control and Prevention Agency.
Traditional anthrax vaccines are made by attenuating Bacillus anthracis or culturing non-pathogenic Bacillus anthracis, which may contain residual toxin components. BARYTHRAX removes this risk and improves vaccine safety.
BARYTHRAX utilizes protective antigen (PA) proteins produced through genetic recombination techniques. With an anthrax infection, PA is a gateway for 2 Bacillus anthracis toxins, lethal factor and edema factor, to enter host cells.
BARYTHRAX vaccination can train and stimulate an immune response to neutralize anthrax by utilizing PA proteins.
Eun-chul Huh, President and CEO of GC Biopharma, commented in a press release on April 9, 2025, "This achievement underscores our commitment to localizing critical medicines for public health and national security. GC Biopharma will continue leading efforts to ensure stable supplies of essential medical products, as we have been doing with other vaccines and blood products since our founding."
Anthrax, caused by Bacillus anthracis, is a class-1 infectious disease capable of surviving extreme conditions and spreading easily through airborne transmission. If untreated, its fatality rate can reach up to 97%, making it a significant threat as a potential biological weapon.
The MFDS's approval, supported by GC Biopharma's production capacity, will pave the way for the company to supply Korea's essential anthrax vaccine reserve.
In the United States, very few people get anthrax from infected animals or contaminated animal products. The U.S. CDC says The type of illness a person develops depends on how anthrax enters the body: through the skin, lungs, or gastrointestinal system.
Getting a vaccine or taking certain antibiotics after exposure to anthrax can help prevent illness.
As of April 9, 2025, the U.S. FDA has approved anthrax vaccines for those at risk of exposure to anthrax bacteria.
For example, CYFENDUS™ is a combination of BioThrax® (anthrax vaccine adsorbed) and CPG 7909, a synthetic short DNA sequence as a vaccine adjuvant.
Shigellosis is the second leading cause of fatal diarrheal disease worldwide, strongly contributing to pediatric morbidity and mortality, without a U.S. FDA-approved vaccine available.
According to public health leaders and the Gates Foundation, developing an effective vaccine to prevent this deadly disease is essential in many areas worldwide.
To address this need, Valneva SE and LimmaTech Biologics AG today announced that the first participant has been vaccinated in a Phase 2 infant safety and immunogenicity study of Shigella4V2 (S4V2), the world's most clinically advanced tetravalent bioconjugate vaccine candidate against shigellosis.
Dr. Juan Carlos, Chief Medical Officer of Valneva, commented in a press release on April 9, 2025, "Seeing so many infants and children dying from shigellosis is not acceptable if it can be prevented with a vaccine."
"As such, the development of Shigella vaccines has been identified as a priority by the World Health Organization and, in line with our mission of developing vaccines against infectious diseases with unmet medical needs, we are focused on delivering a preventative solution against this deadly disease."
In the Phase 2 study S4V02, the safety and immunogenicity of S4V2 will be tested in approximately 110 nine-month-old infants to identify the best dose to be tested in a Phase 3 trial.
Sponsored and conducted by LimmaTech, S4V02 is a randomized, controlled, and blinded study conducted at a single study site in Kenya. Participants will receive a two-dose vaccination with one of two different vaccine dose levels of S4V2 or a control vaccine. Safety will be evaluated throughout the trial for approximately six months following the last vaccination.
Results of the phase 2 study, which is supported by funding from the Gates Foundation, are expected in the second half of 2025.
In November 2024, Valneva and LimmaTech launched a Phase 2b controlled human infection model (CHIM) study of S4V2 in healthy Shigella-naïve adults. This CHIM study forms part of the companies' staggered and risk-mitigating development strategy for S4V2, as it should provide the first results on efficacy before potentially advancing to further CHIM and Phase 3 studies.

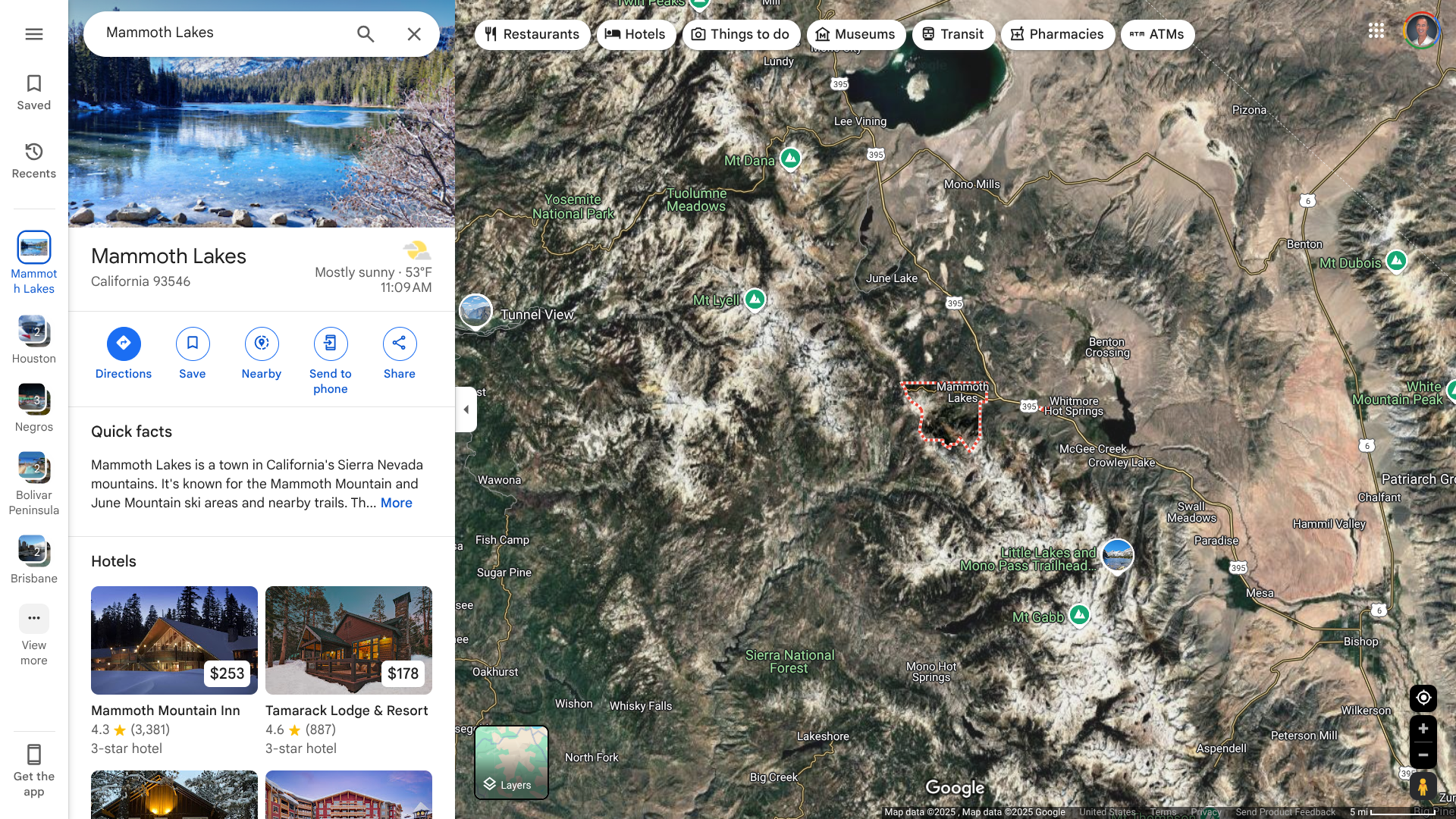
Mono County Public Health has confirmed a third fatality due to Hantavirus in the Town of Mammoth Lakes, a favorite outdoor destination for vacationers in Northeast California.
As of April 3, 2025, Mono County has recorded 27 cases since it was first reported here in 1993, the most in California. Twenty-one of these infections affected county residents, and six occurred among visitors who were infected in Mono. In
As of 2022, 864 cases of Hantavirus disease were reported in the United States.
According to the U.S. CDC, Hantavirus is a serious and often fatal illness contracted through infected deer mouse droppings, urine, or saliva. It most commonly occurs in the late spring or summer.
“A third case of Hantavirus Pulmonary Syndrome (in 2025), each of which has been fatal, is tragic and alarming,” said Dr. Tom Boo, Mono County Public Health Officer, in a press release.
“We don’t have a clear sense of where this young adult may have contracted the virus. The home had no evidence of mouse activity. We observed some mice in the workplace, which is not unusual for indoor spaces this time of year in Mammoth Lakes.
We haven't identified any other activities in the weeks before illness that would have increased this person's exposure to mice or their droppings.
We’ve been aware of this suspected case for weeks, but obtaining testing has taken time. The occurrence of three cases in a short period has me worried, especially this early in the year.
We’ve gone about a month without additional suspect cases, but remain concerned about the increased activity.
We believe deer mouse numbers are high this year in Mammoth (Eastern Sierra). An increase in indoor mice elevates the risk of Hantavirus exposure.
As far as we know, none of these deceased individuals engaged in activities typically associated with exposure, such as cleaning out poorly ventilated indoor areas or outbuildings with a lot of mouse waste. Instead, these folks may have been exposed during normal daily activities in the home or the workplace.
Many of us encounter deer mice daily, and there is some risk. We should pay attention to the presence of mice and be careful around their waste,” stated Dr. Boo.
Without a preventive vaccine available in 2025, the county says to avoid eating food that may have been contaminated by rodents and always wash your hands thoroughly after any potential exposure.