Search API
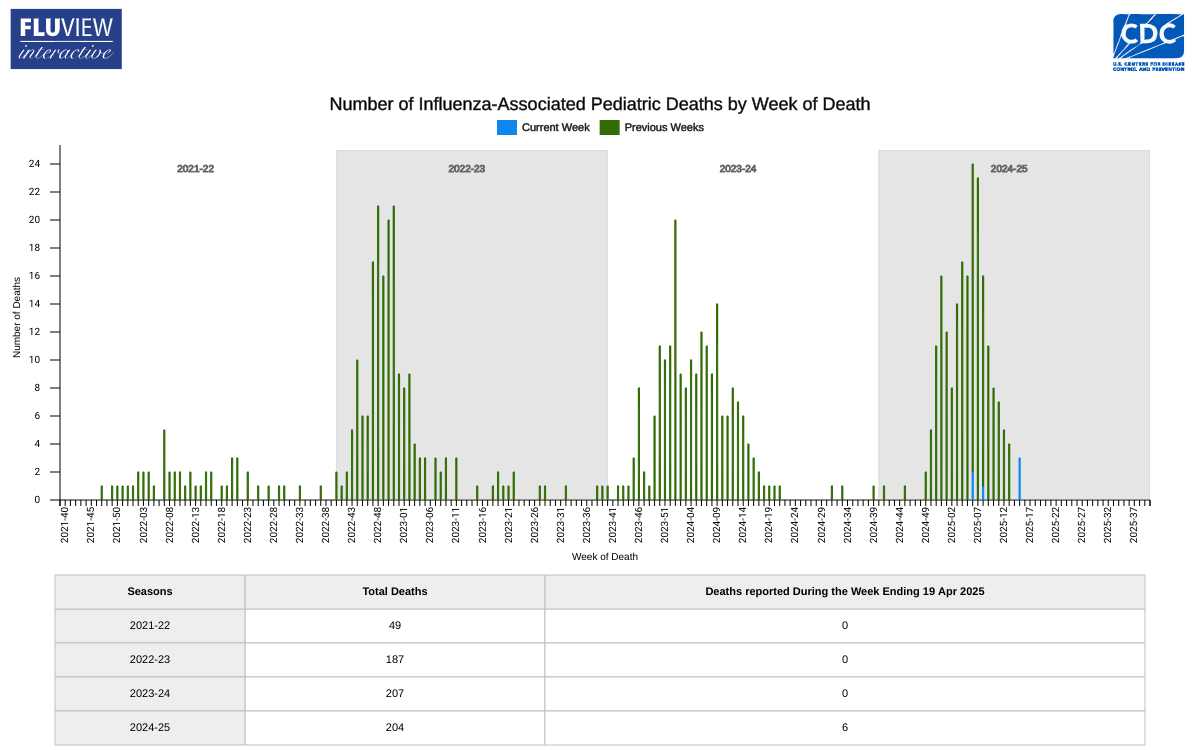
While influenza activity continued to decline across the United States, flu-related fatalities among children increased, now totaling 204 for the 2024-2025 flu season.
This data exceeds the fatality total for each of the last two flu seasons.
The Centers for Disease Control and Prevention (CDC) Influenza Surveillance Report: Key Updates for Week 16, reported on April 25, 2025, that this flu season is classified as a high severity season overall and for all age groups (children, adults, older adults) and is the first high severity season since 2017-2018.
According to the National Center for Health Statistics Mortality Surveillance data, 0.3% of the deaths that occurred during the week ending April 19, 2025 (Week 16), were due to influenza.
This percentage decreased by 0.1% or more compared to Week 15.
The CDC continues to recommend that everyone aged six months and older receive an annual flu vaccine as long as influenza viruses are circulating. As of late April, various flu shots are available at community pharmacies throughout the United States.
Furthermore, suppose your summer vacation plans for 2025 include a trip to the Southern Hemisphere, where the flu season is in full swing. In that case, your pharmacist may recommend an additional flu shot for protection.
Once again, research affirms that healthcare professionals remain the most trusted source of information (73%) and can significantly influence disease prevention efforts, such as vaccination.
A University of Texas Southwestern Medical Center survey published on April 24, 2025, found that 58% of adults say they would be willing to be vaccinated against mpox if their healthcare provider recommended it.
This data represents a 12% increase since 2022.
According to an article published in Vaccine (Volume 56, May 22, 2025, 127141), self-reported knowledge of mpox (40%), as well as perceived self-efficacy (55%) and mean risk perception (3.2), have also increased since the 2022 survey.
Respondents from the Health and Human Services Region 1 (CT, ME, MA, NH, RI, VT) were the least likely to receive the vaccine, even when recommended to do so.
These results highlight the need for ongoing education among adults to improve awareness of and vaccination intention for the mpox vaccine.
The consistently high degrees of trust placed in healthcare professionals and officials should guide future communications about mpox and other infectious diseases, and reinforce the importance of leveraging trusted sources to share essential public health information, wrote these researchers.
In May 2022, an unprecedented global outbreak of mpox Clade IIb prompted the World Health Organization (WHO) to declare a Public Health Emergency of International Concern. Since then, mpox cases, including Clade 1, have been reported in more than 126 WHO Member States.
As of April 28, 2025, mpox vaccination (JYNNEOS) services are commercially offered throughout the United States.

Recent research suggests that a herpes zoster (HZ) vaccine, commonly referred to as the shingles vaccine, may reduce the risk of receiving a dementia diagnosis following vaccination.
According to a study published in JAMA on April 23, 2025, there is evidence of a beneficial effect of herpes zoster vaccination in preventing or delaying dementia, which is more likely to be causal than the associations reported in existing correlational evidence.
In this quasi-experimental study using electronic health record data from Australia, being eligible for herpes zoster vaccination based solely on date of birth significantly decreased the probability of receiving a new dementia diagnosis during 7.4 years by 1.8 percentage points.
A similar study conducted in Wales also showed that HZ vaccination appears to prevent or delay the onset of dementia by about 20%.
These researchers wrote, 'this study and the analysis in Wales provide evidence that is more robust to confounding concerns (eg, healthy vaccinee bias) than is the existing associational evidence.'
In the United States, shingles vaccination services are offered at most pharmacies in April 2025.

When the U.S. Centers for Disease Control and Prevention (CDC) reissued a Global Polio Alert on April 22, 2025, it identified 38 countries, indicating that the spread of poliovirus remains a public health emergency.
But the CDC did not mention Israel, but did include its neighbor, Gaza.
According to the Ministry of Health's Central Virus Laboratory, on April 24, 2025, poliovirus was detected in environmental samples in central Israel and the Jerusalem area, which has about 1 million residents.
However, as of April 27, 2025, Israel's ministry had not reported any recent polio cases.
In March 2023, several children were confirmed with polio in Israel.
Over the past years, Israel has offered polio vaccination services throughout the country. But, as of March 2025, the administration of the live attenuated polio vaccine (oral) has been discontinued.
In its place, the inactivated polio vaccine is now offered. This is the same polio vaccine provided in the United States.
Most African countries have switched to the nOPV2 polio vaccine over the past three years.
The CDC recommends that before travel to any listed destination, adults who previously completed the routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine, which will extend their protection from this severe disease.
Because Zika infection during pregnancy can cause severe congenital disabilities associated with congenital Zika syndrome, the U.S. Centers for Disease Control and Prevention (CDC) advises pregnant women to avoid visiting outbreak areas in 2025, especially during the rainy season.
As of April 27, 2025, the Pan American Health Organization (PAHO) has confirmed 8,392 Zika cases in the Americas this year, led by Argentina and Brazil.
Last year, 42,127 ZIka cases and two related fatalities were confirmed in the Americas.
Within the United States, Puerto Rico has been categorized as having a risk of Zika virus transmission for several years. In 2025, one Zika case was confirmed in this U.S. Territory, and 16 cases were reported in 2024.
On April 23, 2025, the CDC's updated Yellow Book says healthcare professionals in the U.S. should know how to diagnose, treat, and prevent Zika in international travelers, especially pregnant women.
The CDC writes providers should 'carefully evaluate pregnant women with laboratory evidence of Zika virus infection; closely manage these patients during pregnancy and carefully evaluate live-born infants for clinical features associated with intrauterine infection.'
Because Zika and dengue viruses share a similar global geographic distribution and cause infections that can be difficult to differentiate, some state health departments and many commercial laboratories (such as UltaLabs) perform Zika virus nucleic acid amplification testing and IgM testing.
The World Health Organization says developing a safe and efficacious Zika vaccine and monoclonal antibody (mAb) is a global health priority.
However, as of April 2025, the U.S. Food and Drug Administration has not approved any Zika vaccine or mAb. One innovative Zika vaccine candidate is progressing in clinical trials.
Therefore, the best recommendation to prevent Zika is to avoid being bitten by virus-carrying mosquitoes this summer!
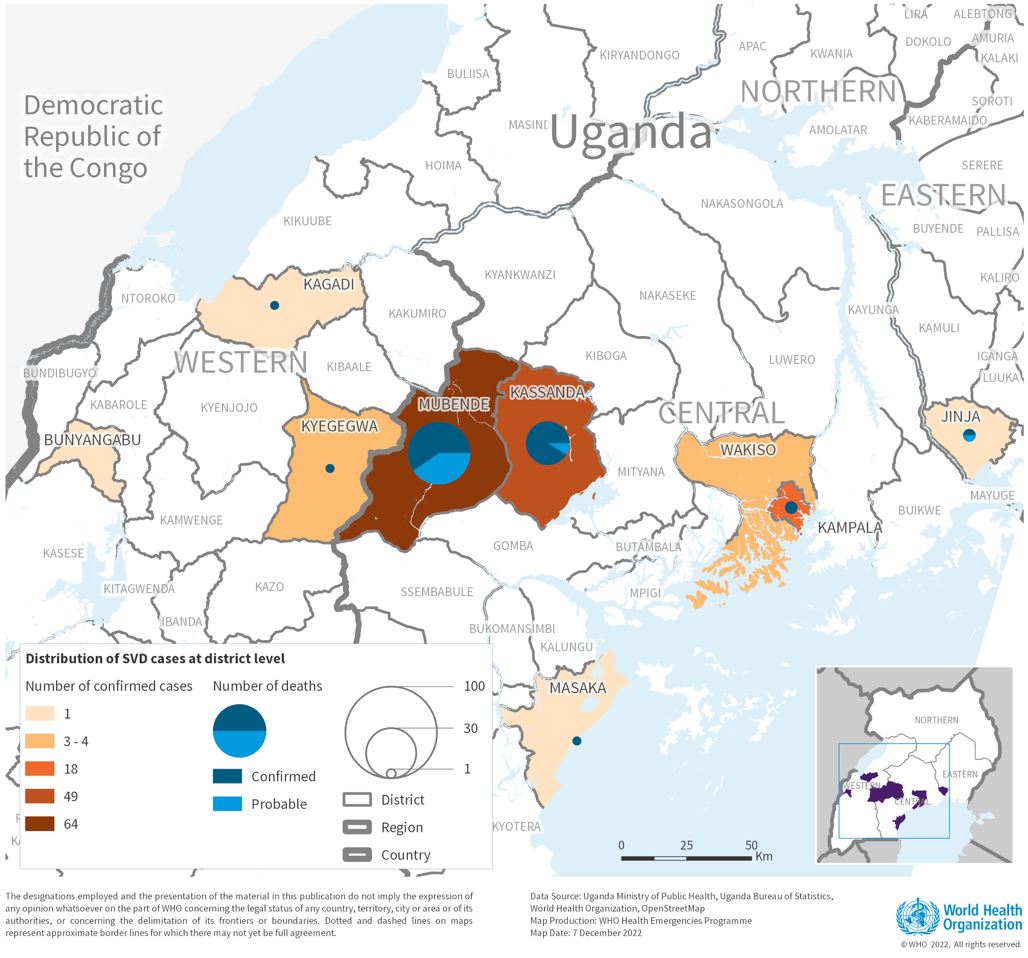
The Republic of Uganda today announced the end of the current Sudan Ebolavirus disease (SVD) outbreak, less than three months after the virus was confirmed in the capital Kampala.
This strain of Ebolaviris often causes a severe, fatal illness in infected people.
As of April 26, 2025, during this outbreak, 14 SVD cases, including 12 confirmed and two probable cases, had been reported.
And four deaths, two confirmed and two probable, occurred.
"This outbreak challenged us in new ways. It touched both urban and rural communities across the country and unfolded against the backdrop of significant global funding constraints," said Dr Chikwe Ihekweazu, Acting WHO Regional Director for Africa, in a press release on April 26, 2025.
"The response demonstrated Uganda's long-standing leadership in tackling public health emergencies. As WHO, we are extremely proud to have supported these efforts every step of the way."
Despite the absence of licensed countermeasures against this species of Ebolavirus, Sudan candidate vaccines are in various phases of clinical trials. Within four days of the government's declaration of the outbreak, a randomized clinical trial for vaccine safety and efficacy using the ring vaccination approach was launched.
In addition, the administration of Remdesivir treatment under the Monitored Emergency Use of Unregistered and Experimental Interventions protocol was initiated.
As of today, various Sudan vaccine candidates are being tested in clinical research,
Previously, Zaire Ebolavirus vaccines and therapeutics had been approved for use in Africa,
In addition to Eboa, Uganda is experiencing polio and mpox outbreaks in 2026. Vaccines for these diseases are commercially available in the U.S.
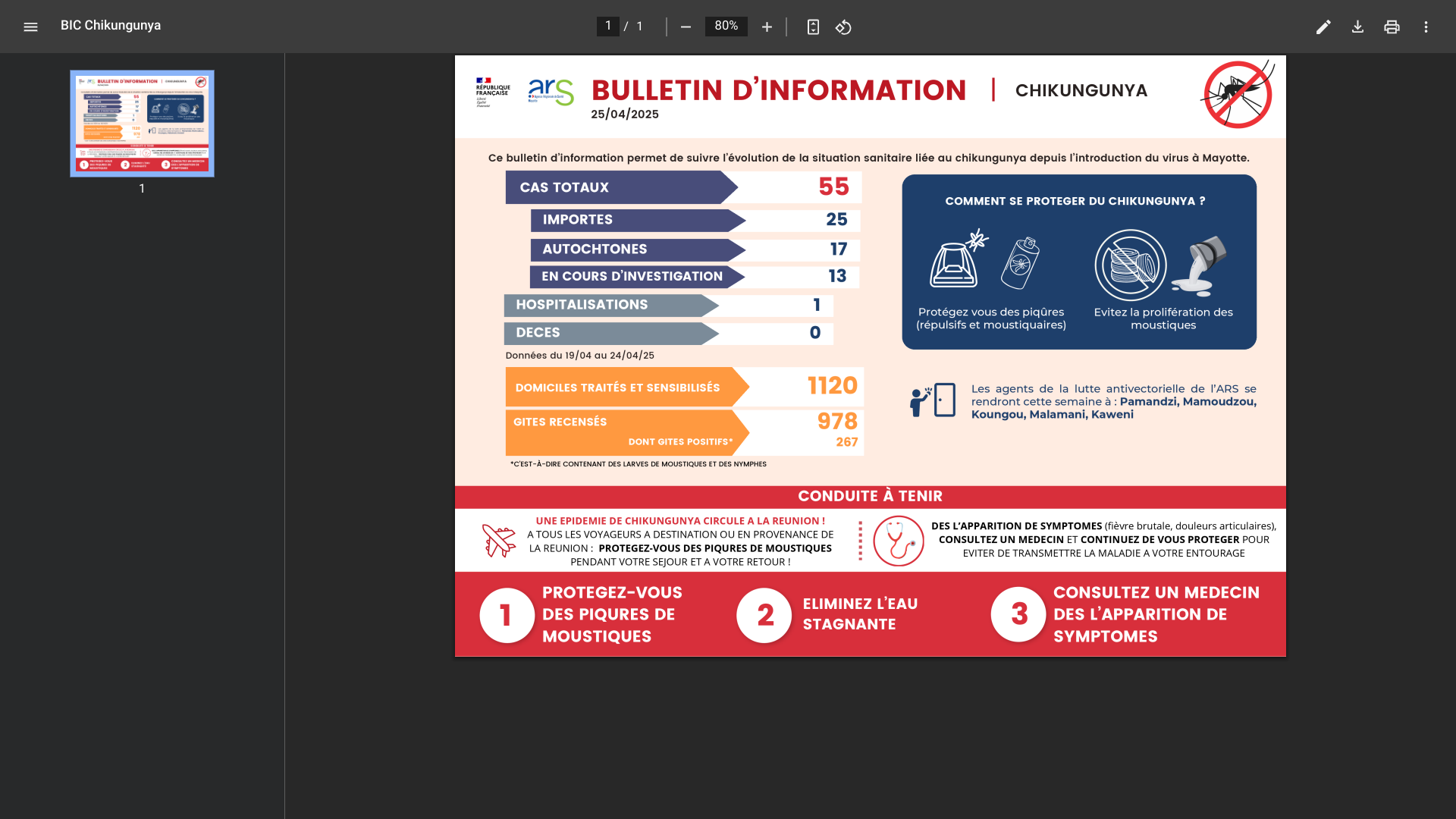
Located in the Chikungunya outbreak in the western Indian Ocean, the French Department of Mayotte announced on April 25, 2025, that 55 indigenous and travel-related cases had been confirmed this year.
The Chikungunya virus is transmitted to people by mosquitoes. In areas where the virus is circulating, particularly in La Réunion and Mauritius, it is essential to protect yourself from mosquito bites, says ARS Mayotte.
Following the identification of the first indigenous (local) Chikungunya case on Mayotte, the Regional Health Agency activated level 2A of the ORSEC plan on March 26, 2025.
Since the, preventive vaccination services have been offered on the island. ARS Mayotte has published a map that identifies locations.
Currently, Valneva SE's IXCHIQ® Chikungunya vaccine is being administered. Vaccination remains open to people aged 18 to 64 with comorbidities.
Like all medicines, vaccines can cause side effects; however, these are not serious in the vast majority of people and typically disappear spontaneously within a few days. ARS Mayotte says healthcare providers are available to answer any questions.
The health agency advises, 'People traveling to areas where chikungunya is circulating are asked to apply preventive measures throughout their stay (in Mayotte) and for up to three weeks after their return to avoid transmitting the disease to those around them.'
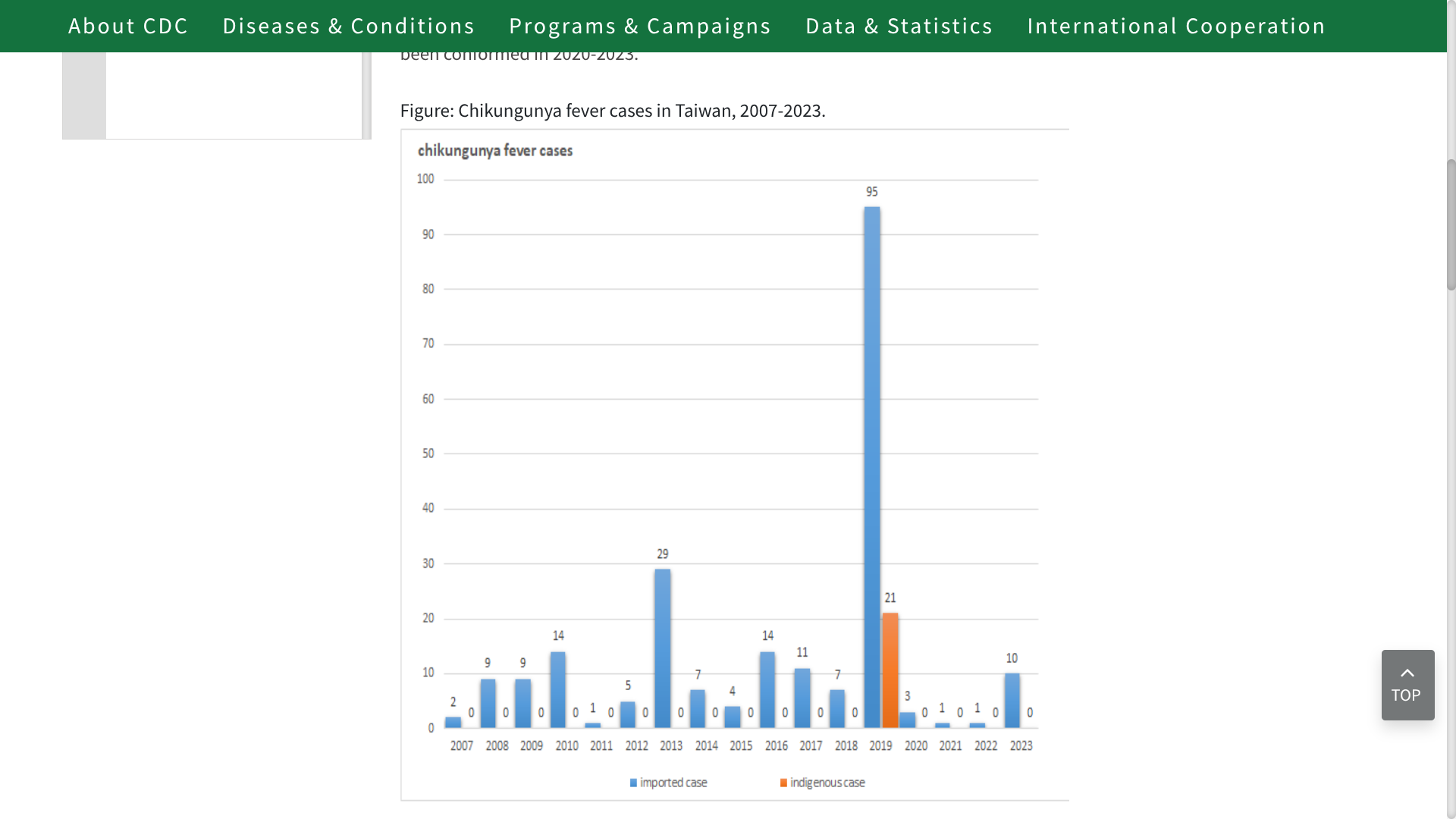
Since Chikungunya was listed as a notifiable infectious disease by the Taiwan Centers for Disease Control (TCDC) in October 2007, a few travel-related and locally acquired cases have been reported most years.
As of April 25, 2025, the TCDC reports 11 Chikungunya cases this year, indicating a potential increase over last year's total of 20 cases.
And may approach 2019's record when 116 total cases were confirmed.
Previously, to identify infectious travelers, fever screening was conducted at the Taiwan Taoyuan International Airport.
The TCDC states that Chikungunya fever is rarely fatal; however, some patients may experience severe joint pain for several weeks, months, or even years.
In the Asia Region, Chikungunya outbreaks have been recorded as early as 1824 in India.
As of April 25, 2025, the World Health Organization reports that Chikungunya transmission has occurred in approximately 110 countries, many of which are in the Americas, including Argentina and Brazil.
From a health perspective, this mosquito-transmitted disease can be prevented with U.S. Food and Drug Administration-approved vaccines, which are commercially available at travel clinics and pharmacies in the United States.
With the continued overlapping incidence of chikungunya, dengue, malaria, Zika, and yellow fever diseases in the Region of the Americas, should international travelers be vaccinated before arriving in Florida?
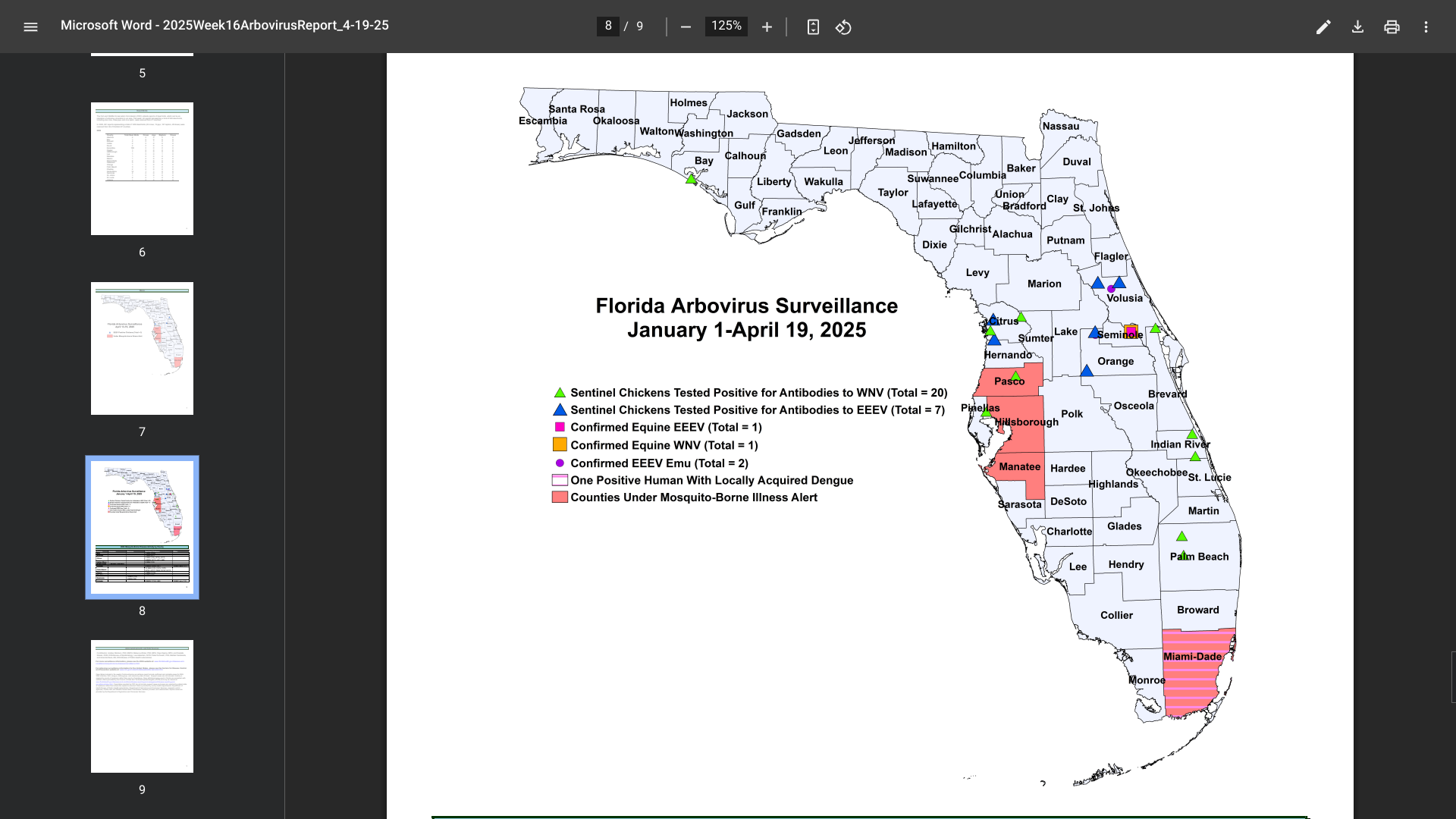
According to the Florida Department of Health (FDH) Arbovirus Surveillance update #16, dated April 19, 2025, numerous travelers have been diagnosed with vaccine-preventable diseases this year, particularly in the Miami and Tampa areas.
To notify people of these health risks, FDH has missed alerts for Hillsborough, Manatee, Miami, and Pasco counties in 2025.
For example, last year, 11 instances of chikungunya were reported in individuals with a travel history to Brazil (five), India (five), and Pakistan.
In 2025, sixty cases of dengue fever had already been reported among individuals who had traveled internationally, and one locally acquired case of dengue (DEN-3). During 2024, 1,016 travel-associated dengue cases were reported, primarily among visitors from Brazil, Cuba (567), and Puerto Rico.
Furthermore, 91 locally acquired dengue cases were reported from ten counties, including Miami-Dade (50), in 2024.
In 2024, 72 cases of travel-related malaria were reported in individuals with a history of visiting malaria-endemic areas, such as Africa (Nigeria) and Central and South America.
As of April 25, 2025, the U.S. Centers for Disease Control and Prevention, Canada Health, and the United Kingdom have not issued vaccination requirements for visiting Florida.
Of these mosquito-transmitted diseases, chikungunya vaccines are commercially available at most pharmacies and are recommended by various health agencies.
Merck today announced its financial results for the first quarter of 2025, which included a significant decrease in sales of its cancer prevention human papillomavirus (HPV) vaccines.
On April 24, 2025, Merck reported that GARDASIL/GARDASIL 9 vaccine sales declined 41% to $1.3 billion in 2025.
This decline is primarily due to lower demand in China, partially offset by higher demand in most international regions, particularly in Japan, as well as higher pricing and demand in the U.S.'
'Excluding China, sales grew 14%, or 16% excluding the impact of foreign exchange.'
Robert M. Davis, chairman and chief executive officer, Merck, commented in a press release, “We are working with focus and urgency to both realize the full potential of our near-term opportunities and to rapidly progress the next wave of innovation that will positively impact the lives of patients and drive future value creation for all of our stakeholders.”
The GARDASIL-9 vaccine remains the leading HPV vaccine in the United States, recommended by the U.S. CDC, and offered at most pharmacies.
