Search API
The World Health Organization (WHO) today published the 51st situation report for the multi-country outbreak of mpox, including an update on the epidemiological situation for mpox in Africa, with data as of late April 2025.
This year, the WHO has confirmed 137,892 mpox cases, 317 related fatalities, from 132 reporting countries.
The WHO stated on April 29, 2025, 'Wherever mpox outbreaks are not quickly contained and human-to-human transmission is not stopped, they continue to represent a potential risk of sustained transmission in the community.'
Highlights from this report include, but are not limited to, the following:
Cases of mpox due to clade Ib monkeypox virus (MPXV) continue to be reported primarily in Africa, where eleven countries have reported community transmission of this strain in the past six weeks, as person-to-person transmission has occurred through various means during this outbreak.'
Currently, Uganda is reporting the highest number of confirmed mpox cases globally, with 200 to 300 new cases reported per week. To date, the country has detected only clade Ib MPXV.
The Democratic Republic of the Congo (DRC) continues to report the highest number of cumulative confirmed mpox cases in Africa in 2025, despite a decrease in the number of confirmed cases reported in recent weeks, likely due to a reduction in testing and confirmation capacity. Clades Ia and Ib MPXV continue to circulate in the DRC.
This report provides an overview of mpox vaccination in countries in the African Region, where to date more than 662,000 doses of Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccines have been administered in seven countries.
From the total number of doses, 88% have been administered in the DRC, where the vaccination strategy is being revised in light of the limited vaccine supply.
In the United States, there is an ample supply of the JYNNEOS vaccine, which is recommended by the U.S. CDC for specific individuals when visiting mpox outbreaks in 2025. This mpox vaccine is commercially available in the U.S. at clinics and pharmacies.

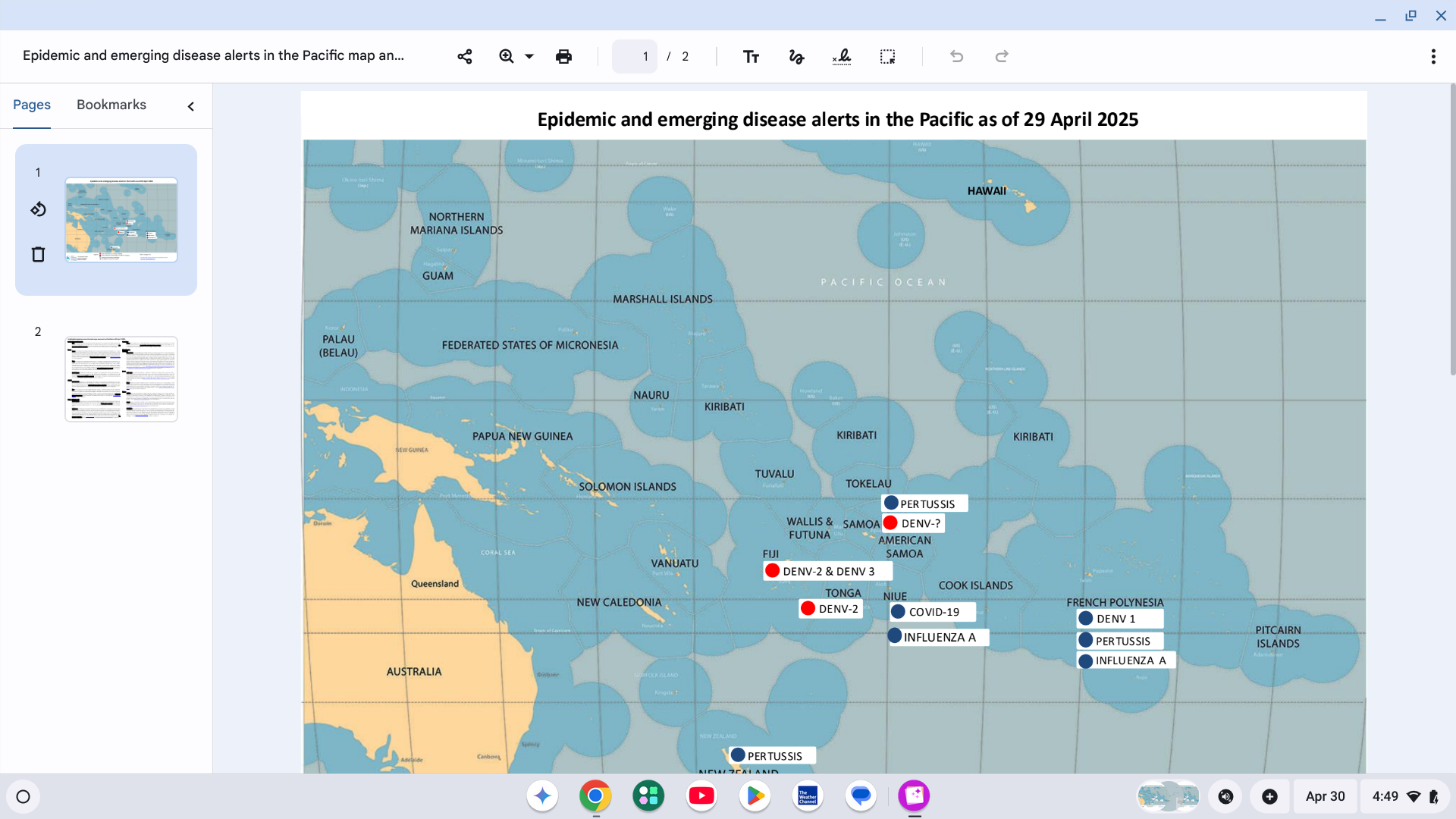
With over 100 countries reporting Dengue fever cases this year, several governments in the Pacific Region are missing alerts for this mosquito-transmitted disease.
For example, the Government of Samoa's Ministry of Health has officially declared a Dengue outbreak in 2025. There has been a significant increase in the number of dengue cases, particularly in Upolu, over the past two weeks.
As of April 17, 2025, the Ministry has reported 15 Fengue cases and one reported death this year.
Samoa has issued a red alert for Dengue of undetermined serotype.
The Ministry of Health is actively monitoring the situation, strengthening response efforts, urging the public to take preventive measures against mosquito bites and to seek medical attention if symptoms appear.
On April 15, 2025, the U.S. CDC reissued a Level 1 Global Dengue Advisory, which identified several countries in the Pacific Region. The CDC recommends avoiding mosquito bites as a way to prevent Dengue infection.
Additionally, several countries in the Pacific Region offer access to Dengue vaccination services in 2025.
The ongoing chikungunya epidemic in France's Réunion Department has resulted in 39,532 cases and nine associated deaths since the beginning of 2025.
This includes 4,304 new cases from April 7 to April 13, 2025.
On April 23, 2025, ARS Réunion stated that 'the chikungunya epidemic continues across the island. Although the number of chikungunya-related primary care consultations is stabilizing, the number of emergency room visits is increasing.'
Local health authorities, the World Health Organization, and the U.S. Centers for Disease Control and Prevention (CDC) are urging heightened vigilance for travelers to Réunion.
Visitors are advised to take preventive measures against mosquito bites, such as using insect repellent, wearing long-sleeved clothing, and staying in accommodations with screened windows or air conditioning.
Additionally, international travelers may pose a risk of introducing chikungunya to other regions where Aedes mosquito vectors are present. It is recommended that they monitor for symptoms, such as fever, joint pain, and rash, for up to two weeks and seek medical attention if symptoms arise.
The CDC says if you are pregnant, consider reconsidering travel to the affected states, especially if you are nearing the delivery of your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
Newborns infected in this way or by a mosquito bite are at risk for severe illness, including poor long-term outcomes.
The vaccination campaign, which started on April 7, 2025, is available for individuals aged 18 to 64 with comorbidities. Health authorities emphasize the importance of monitoring for allergic reactions within 72 hours of vaccination and seeking immediate medical attention if any symptoms occur.
The CDC's Level 2 Travel Advisory recommends vaccination against chikungunya for adults traveling to a destination with a current chikungunya outbreak. The vaccine should not be administered to individuals with weakened immune systems or those who have experienced a severe allergic reaction to any component of the vaccine.
In the United States, chikungunya vaccination services are available as of April 30, 2025, at travel clinics and pharmacies.
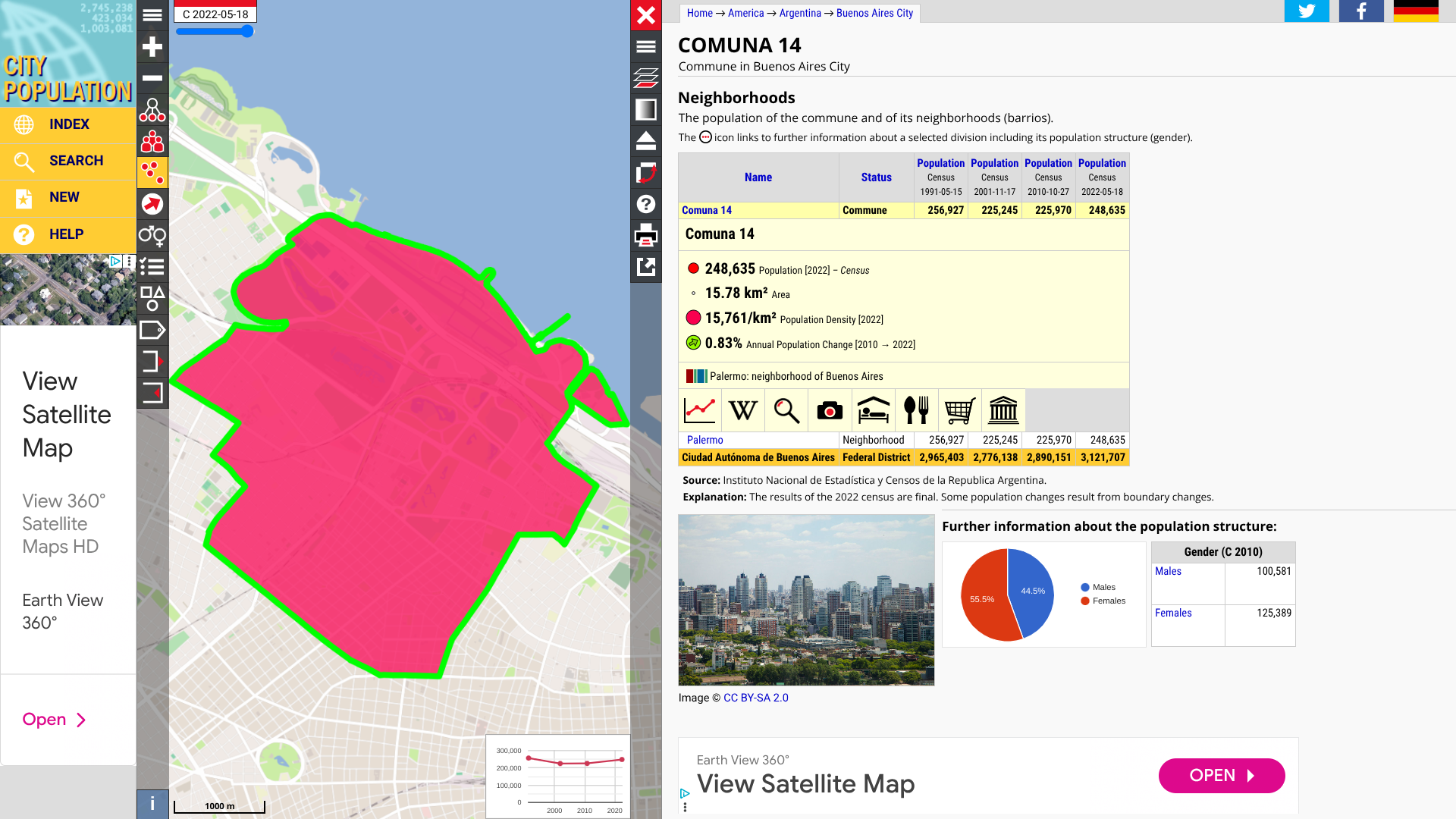
According to the Pan American Health Organization (PAHO), a total of 21 measles cases have been confirmed in the Autonomous City of Buenos Aires and the Province of Buenos Aires, Argentina.
As of April 16, 2025, three of these measles cases were imported and 18 were locally acquired.
The Buenos Aires National Health Service has reported that the majority of cases are concentrated in Commune 14, which is home to approximately 248,000 people.
The PAHO stated that the index case of this outbreak was reported in January 2025 in a child with a history of international travel.
Twelve of the cases were confirmed with genotype B3, and one of the imported cases, associated with recent international travel to Thailand, was identified as genotype D8.
Throughout 2025, a total of 2,318 measles cases, including three deaths, have been confirmed in six countries in the Region of the Americas, which includes the United States.
This PAHO data represents an 11-fold increase compared to the same period in 2024.
The PAHO wrote 'Measles is a highly contagious, airborne viral disease that can lead to severe complications and death. This disease is preventable with two doses of the MMR vaccine, which is highly recommended for most people.
As of April 29, 2025, the regional risk is currently assessed as high, while the global risk remains moderate.'
However, the Argentine Republic was not included in the U.S. CDC's recent Global Measles Advisory that identified 58 countries reporting measles outbreaks.
The Yellow fever outbreak in South America now includes the Republic of Ecuador, home to approximately 17 million residents.
As of April 28, 2025, Ecuador's Public Health Minister, Edgar Lama, announced that there are three confirmed cases, with a fourth case under investigation.
The first case was detected in the province of Zamora Chinchipe, located at the southeastern end of the Amazon Basin.
Furthermore, Minister Lama stated in a media release, "We remain vigilant and are not in any situation similar to Colombia and Peru, which have had so many cases of yellow fever," he stated.
As of the end of April 2025, yellow fever outbreaks have been reported throughout South America.
Ecuador is a popular tourist destination that features the Galápagos Islands in the Pacific Ocean.
To alert international travelers to the Yellow fever health risk, the U.S. CDC states that proof of vaccination is not required for direct travel from the United States, or when traveling to areas above 7,550 ft in elevation, or the cities of Guayaquil or Quito, or the Galápagos Islands.
However, the CDC has included Ecuador in recent Dengue and Oropouche in Level 1 Travel Health Advisories.
In the United States, yellow fever vaccination appointments are offered at certified travel clinics and pharmacies.
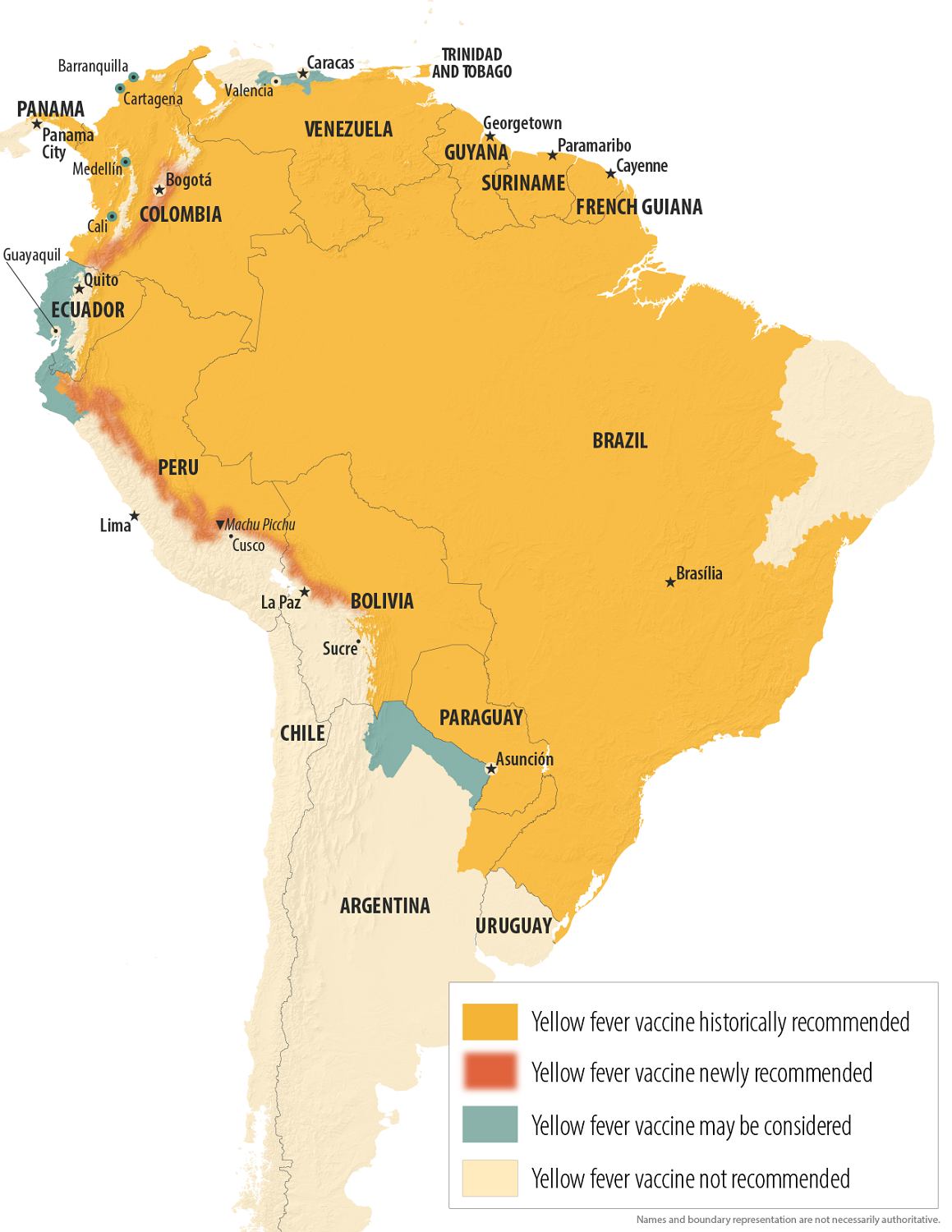
In the Republic of Peru, the sudden upswing in Yellow fever cases has caused outbreak alerts to be issued for this western South American country in 2025.
As of April 25, 2025, 32 human cases of yellow fever have been confirmed, including eleven fatal cases.
Last year, Peru reported 19 confirmed cases of yellow fever, resulting in nine deaths for the entire year.
In 2025, cases were confirmed in most Peruvian departments, with the district of Imaza reporting the highest number of cases (12, including three fatal cases).
Most of the confirmed cases were males (90.6%), with a history of exposure in wild and/or wooded areas, due to agricultural work activities.
Even though the Yellow fever vaccination is recommended, 71.8% of the cases had no history of vaccination against yellow fever.
According to the U.S. CDC, vaccination is not recommended for travel to areas with elevations greater than 7,550 ft, including specific regions west of the Andes, the city of Lima, and the highland tourist areas of Cusco, the Inca Trail, and Machu Picchu.
These Peurivan destinations welcome over 1 million people annually.
When departing for Peru in 2025, travel clinics and pharmacies offer a specific recommendation for the yellow fever vaccination.
While most hikers and campers await the results of a Phase 3 clinical trial of the most advanced Lyme disease vaccine candidate, The Lancet Infectious Diseases recently published an article highlighting very positive data from the Phase 2 study.
On April 25, 2025, results from the NCT04801420 study confirm the previously observed safety and immunogenicity profiles of VLA15 in adults and extend them to children aged five years and older, as well as adolescents.
Additionally, the greater immunogenicity of VLA15 among children and adolescents might translate to increased flexibility in the real-world clinical setting.
According to Vaneva SE and Pfizer Inc., VLA15 is an investigational multivalent protein subunit vaccine that utilizes an established mechanism of action for a Lyme disease vaccine, targeting the outer surface protein A (OspA) of Borrelia burgdorferi, the bacterium that causes Lyme disease.
OspA is a surface protein expressed by the bacteria when present in a tick. Blocking OspA inhibits the bacterium’s ability to leave the tick and infect humans.
The vaccine candidate covers the six most prevalent OspA serotypes expressed by the Borrelia burgdorferi sensu lato species in North America and Europe.
Lyme disease, if left untreated, can cause serious chronic complications affecting the skin, joints, heart, or nervous system.
According to various health agencies, the medical need for vaccination against Lyme disease is steadily increasing as the geographic footprint of the disease widens in the United States and Europe.
Throughout Europe, the bacteria are only transmitted by the bites of the ticks Ixodes ricinus and I. persulcatus. In the most affected regions, tick infection rates may exceed 10%. These areas are primarily located in central Europe.
However, the ECDC says in recent years, the spread of infected ticks has extended toward northern latitudes, including Scandinavia.
In the United Kingdom, the South of England and the Scottish Highlands have been earmarked by the government as high-risk areas for Lyme disease in 2025. Annually, the UK Health Security Agency reports about 1,500 laboratory-confirmed cases of Lyme disease.
With millions of people returning to the woods and mountains this summer, analysts say there is significant pent-up demand for an effective Lyme disease vaccine.