Search API
As the global number of Dengue cases began to mount in 2025, several countries in the Pacific Region, such as the Republic of Singapore, are reporting weekly increases.
The island country and city-state of Singapore in Southeast Asia has recorded several recent Dengue outbreaks.
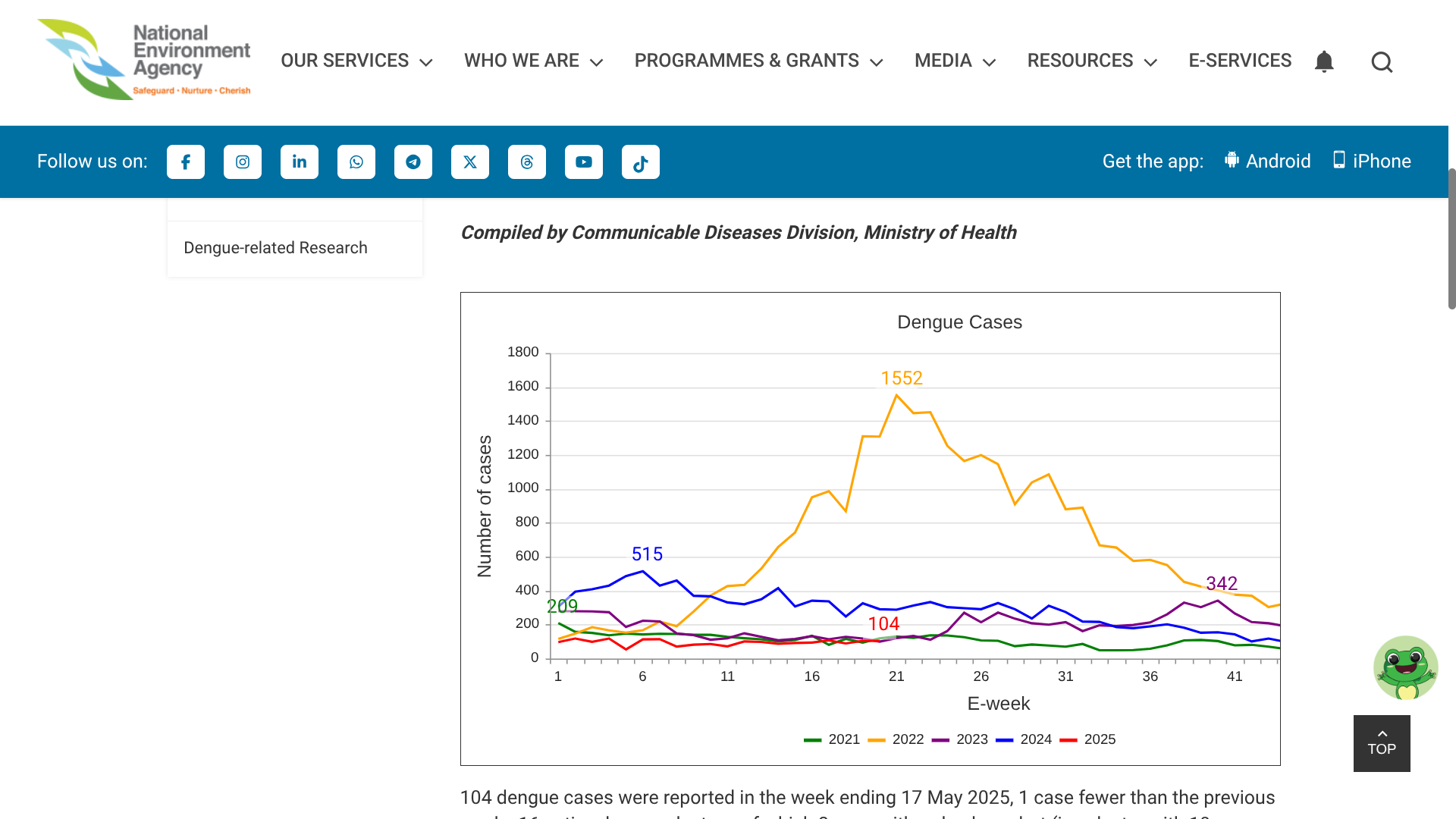
As of May 20, 2025, Singapore's National Environment Agency (NEA) wrote, 'Collective community action and vigilance are critical to help prevent a surge in Dengue cases this year.'
NEA reported 104 Dengue cases in the week ending May 17, 2025, 1 case fewer than the previous week.
Furthermore, there are 16 active Dengue clusters, of which there were with red alerts,
Persistent transmission has been noted in the cluster at Hougang Avenue 1 (106 cases). The large clusters at Begonia Drive/Dedap Road (33 cases) and Woodlands Avenue 1/Woodlands Street 31 (30 cases) have relatively fast transmission rates, with an increase of 10 and 12 cases, respectively, from the previous week.
Among the four Dengue virus serotypes circulating in Singapore, Dengue virus serotype 2 has been predominant in Singapore since September 2023.
Globally, data indicates that over 2.5 million Dengue cases and 1,305 related fatalities have already occurred in 2025, with the U.S. reporting 1,760 travel-related Dengue cases and one local case this year.
While a second-generation Dengue vaccine is available in about 40 countries, it is unavailable in Singapore and the United States in 2025.
The U.S. CDC does recommend various routine and travel vaccinations before visiting Singapore.
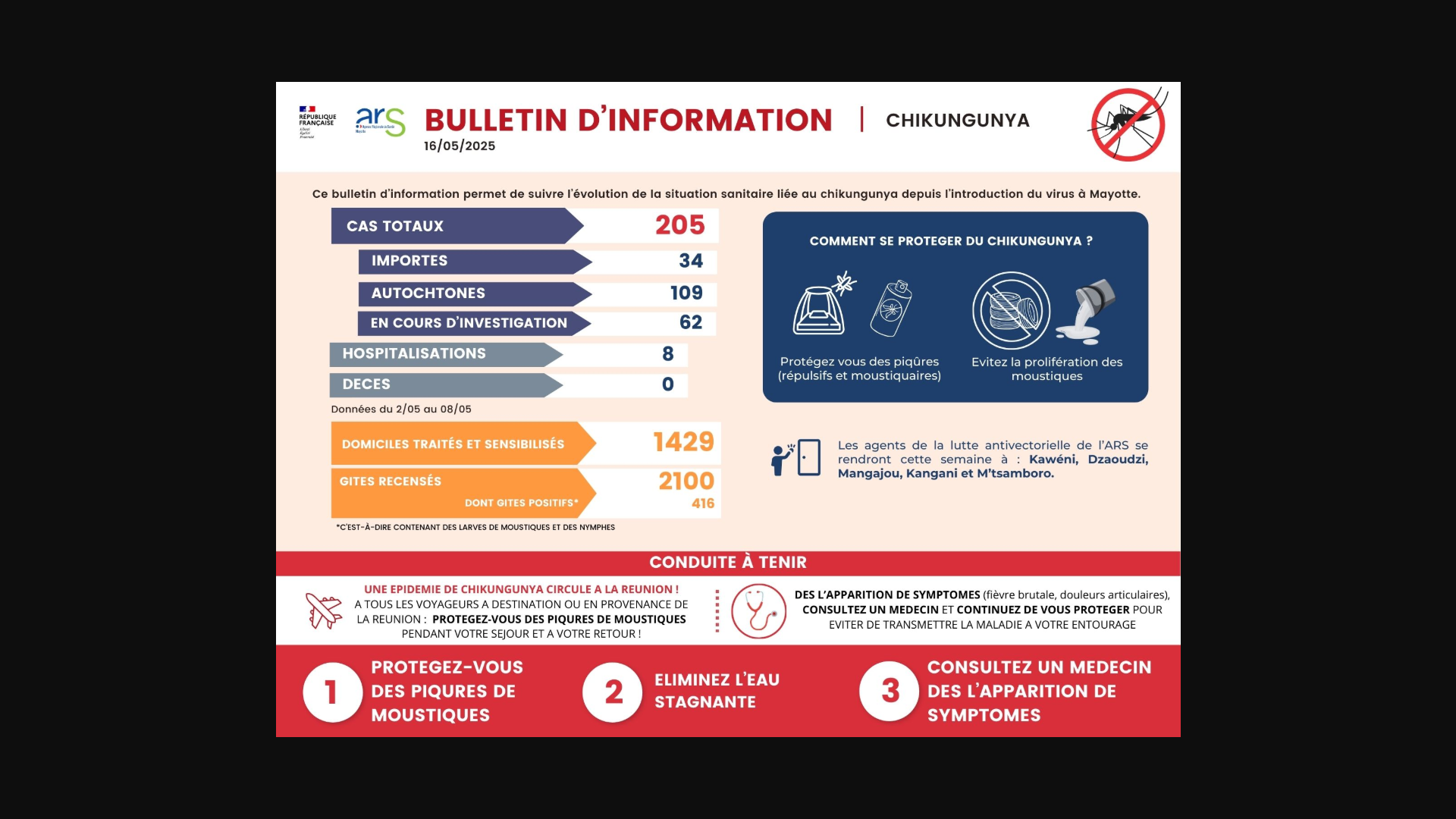
As the Chikungunya virus outbreak continues in the Indian Ocean countries, the French Department of Mayotte has become another hotspot for this mosquito-transmitted, vaccine-preventable disease.
As of May 16, 2025, Regional Health Agency (ARS) Mayotte reported 205 confirmed Chikungunya cases, including 34 imported cases, 109 locally acquired cases, and 62 cases currently under investigation.
From a severity perspective, these infections have led to eight hospital admissions during this year's outbreak.
'Given the evolving situation and the risk of an epidemic in the coming weeks, Mr. Sergio Albarello, Director General of ARS Mayotte, in consultation with Mr. François-Xavier Bieuville, Prefect of Mayotte, has decided to activate today the transition to level 2B of the ORSEC plan,' wrote ARS Mayotte.
Furthermore, ARS says it is also possible to get vaccinated against Chikungunya in Mayotte at various locations. Valneva's IXCHIQ® Chikungunya vaccine is recommended to combat severe forms of the disease for people aged 18 to 64 with comorbidities. The latter is available upon medical prescription and free of charge for these people.
In the United States, the CDC recommends that certain international travelers be vaccinated before visiting Chikungunya outbreak areas in 2025.
Member States of the World Health Organization (WHO) today formally adopted the world's first Pandemic Agreement.
Announced on May 20, 2025, the decision by the 78th World Health Assembly culminated in more than three years of negotiations launched by governments in response to the devastating impacts of the COVID-19 pandemic.
Regarding national sovereignty, Agreementent states that: "Nothing in the WHO Pandemic Agreement shall be interpreted as providing the Secretariat of the World Health Organization, including the Director-General of the World Health Organization, any authority to direct, order, alter or otherwise prescribe the national and/or domestic law, as appropriate, or policies of any Party, or to mandate or otherwise impose any requirements that Parties take specific actions, such as ban or accept travellers, impose vaccination mandates or therapeutic or diagnostic measures or implement lockdowns."
The new Agreement was driven by the goal of making the world safer and more equitable in response to future pandemics, such as Disease X.
According to the WHO, Disease X represents the knowledge that a serious international epidemic could be caused by a pathogen currently unknown to cause human disease.
On April 4, 2025, the WHO's Global Health Emergency Corps announced a framework designed to strengthen countries' emergency workforce, coordinate the deployment of surge teams and experts, and enhance collaboration between governments, which was tested.
Disease X was first included in the WHO Blueprint for Epidemics in February 2018, aiming to accelerate the development of medical countermeasures. It is expected to be caused by a "pathogen X," likely a zoonotic disease or infection that can be transmitted between humans and animals.
In the United States, the National Institutes of Allergy and Infectious Diseases (NIAID) developed a Pandemic Preparedness Plan to prepare for future public health emergencies caused by infectious diseases.
While it is recognized that pathogens other than viruses could lead to public health emergencies, the NIAID Pandemic Preparedness Plan focuses on viruses that could cause epidemics or pandemics.

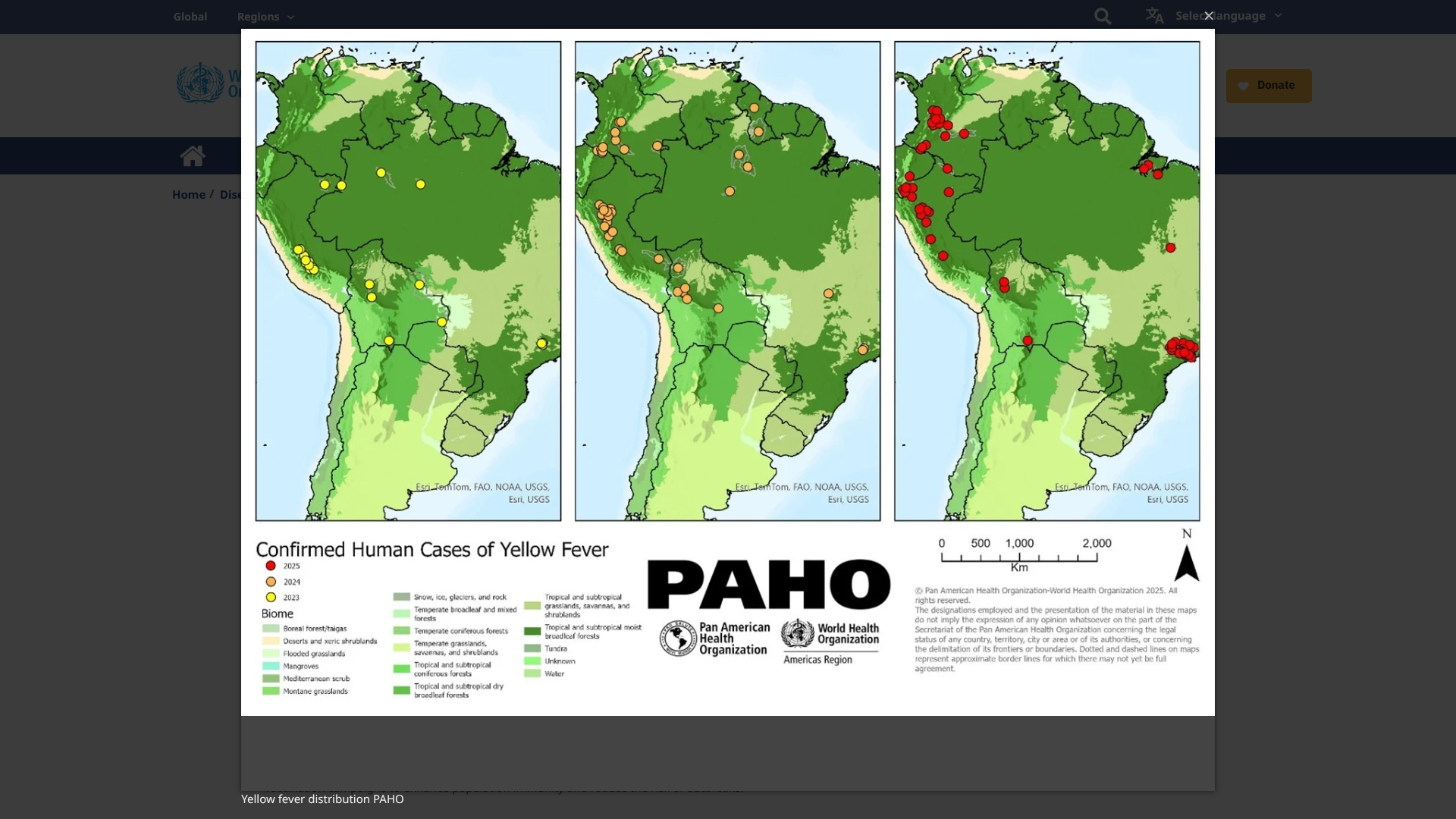
Researchers believe the yellow fever virus has existed in the Region of the Americas since the 17th century and is now considered one of the most dangerous mosquito-transmitted infectious diseases.
According to the World Health Organization (WHO, DON570), as of May 16, 2025, the YF virus has become endemic in tropical areas of 13 countries in the Americas.
During 2025, five countries in the Americas reported 212 confirmed human cases of yellow fever, including 85 deaths (CFR 40%).
The YF cases were reported in the Plurinational States of Bolivia, Brazil, Colombia, Ecuador, and Peru.
The WHO writes that the 'occurrence of yellow fever outside of the Amazon basin contributes to the overall classification of YF risk in the Americas, especially in endemic countries, as high.'
WHO emphasizes that vaccination remains the primary means for preventing and controlling yellow fever.
The WHO continues to support countries in expanding Sanofi Pasteur YF-VAX® vaccination coverage through routine immunization programs and mass vaccination campaigns, enhancing population immunity and reducing the risk of outbreaks.
In the United States, the CDC recommends yellow fever vaccination at least ten days before visiting outbreak areas. YF vaccination appointments are at travel clinics and pharmacies in May 2025.
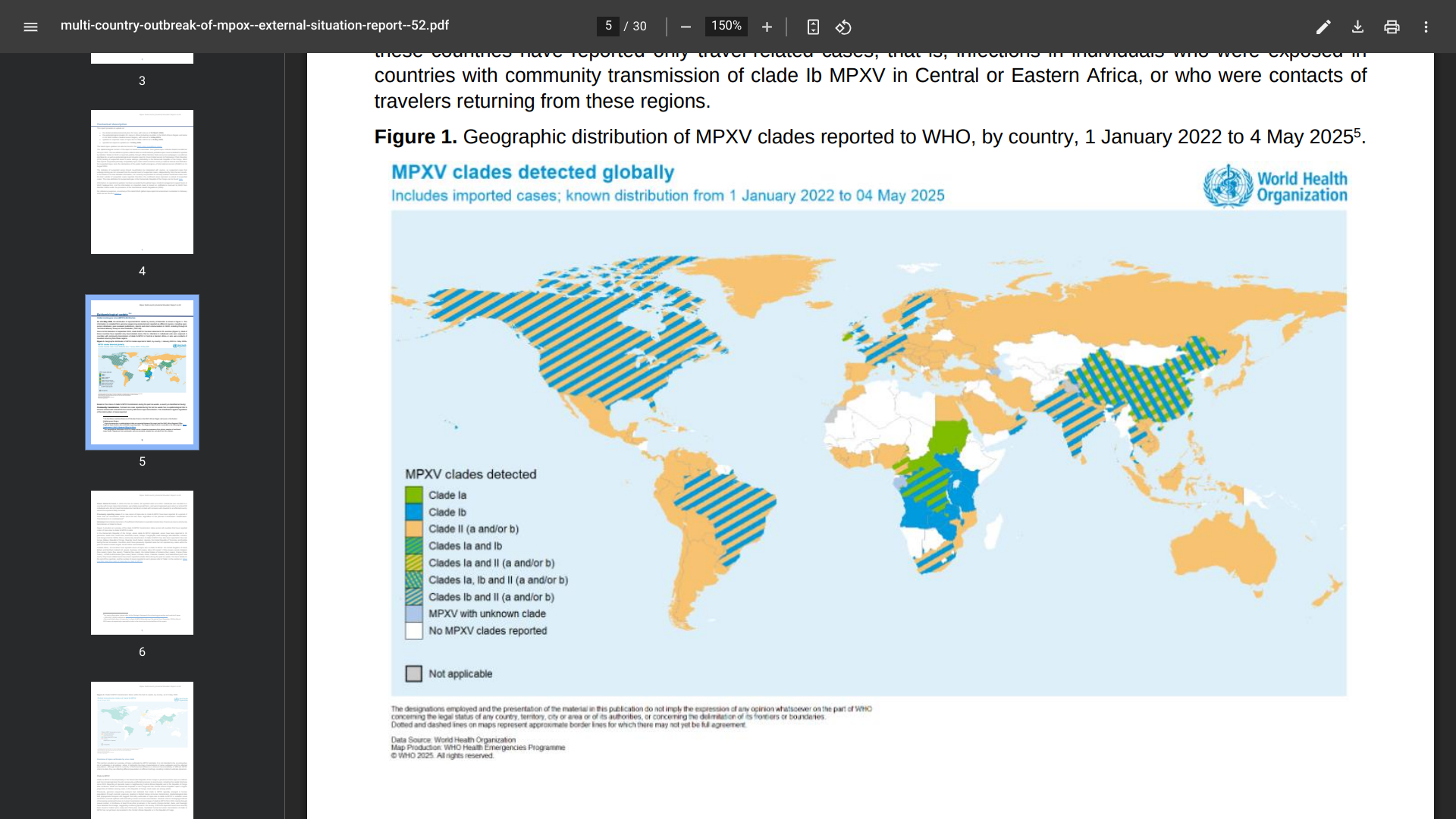
India has retrospectively reported ten cases of mpox due to clade Ib mpox virus detected between December 2024 and March 2025.
According to the WHO's Multi-country outbreak of mpox, External situation report #52, as of May 14, 2025, all clade 1b cases reported in India had a recent history of travel to countries in the Gulf or contact with travellers from those countries.
Outside Africa, 16 countries have reported travel-related cases of mpox due to clade Ib: the United Kingdom of Great Britain and Northern Ireland (12 cases), Germany (10 cases), China (seven cases), Belgium (five cases), Qatar (five cases), Thailand (five cases), the United States of America (four cases).
Globally, Cases of mpox due to clade Ib continue to be reported primarily in Africa, where ten countries have reported community transmission of this strain, reaching over 14,000 cases in 2025.
Reported #52 discloses that mpox vaccination in countries in the African Region has more than 668,000 doses of MVA-BN (Bavarian Nordic A/S JYNNEOS®) vaccines administered in seven countries.
From the total number of JYNNEOS doses, 87% have been administered in the Democratic Republic of the Congo.
In the United States, the JYNNEOS vaccine is commercially available at many pharmacies.
The U.S. Food and Drug Administration (FDA) initially approved JYNNEOS for smallpox prevention in September 2019. And on March 31, 2025, the FDA approved the freeze-dried formulation of JYNNEOS.
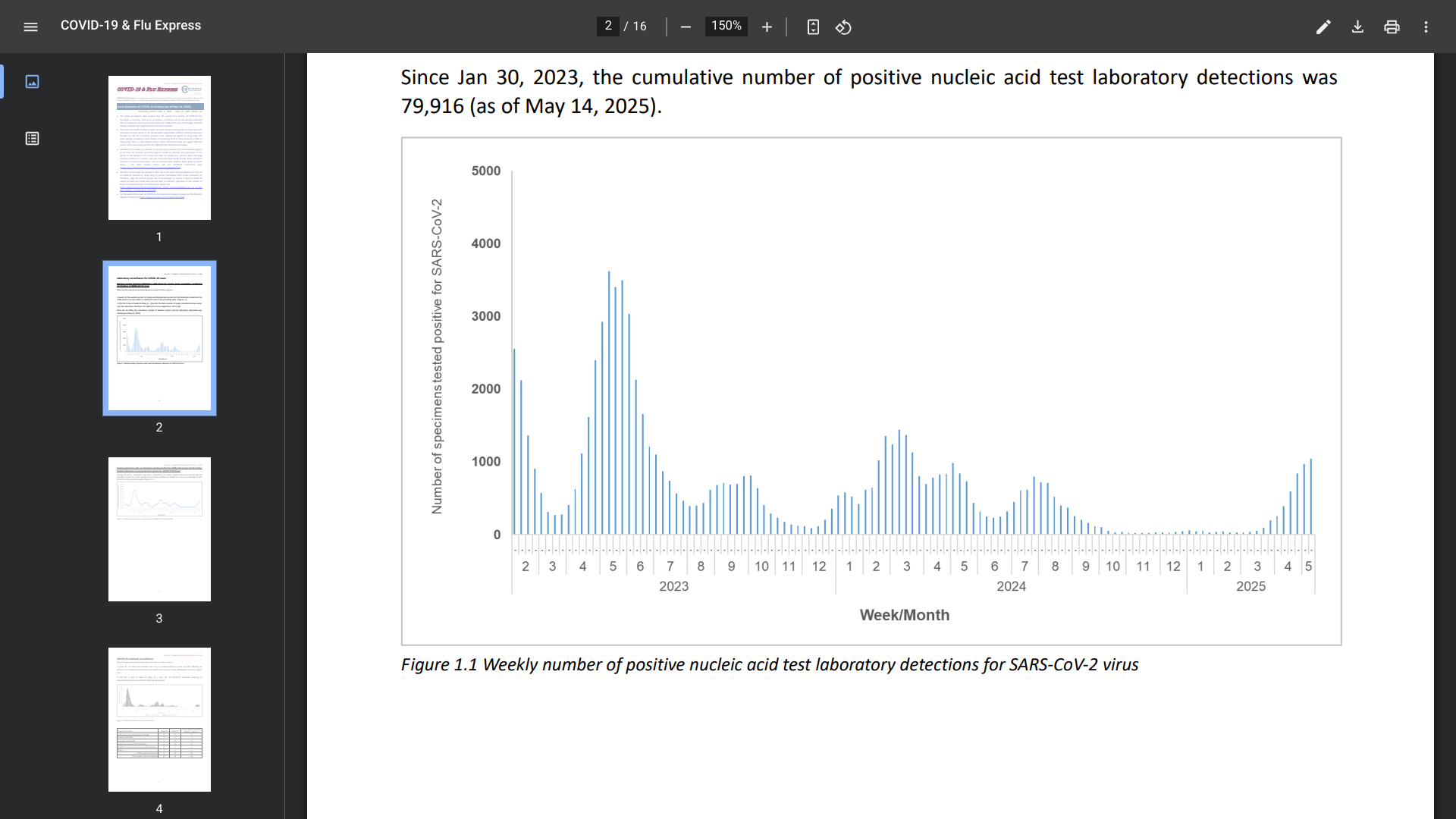
The latest surveillance data (Week #19) from Hong Kong showed that the overall local activity of COVID-19 has continued to increase, with some surveillance indicators having surpassed their highest levels in the past year.
In week 19, the weekly number of newly recorded positive nucleic acid test laboratory detections for the SARS-CoV-2 virus was 1,042, compared to 972 in the preceding week.
And COVID-19 outbreaks occurring in schools/institutions affected 52 people last week.
Furthermore, since January 2023 and May 10, 2025, the cumulative number of fatal cases with cause of death preliminarily assessed to be related to COVID-19 was 1,437.
As of May 14, 2025, the Centre for Health Protection (CHP) has been closely monitoring the local prevalence of SARS-CoV-2 variants in the neighboring regions of Hong Kong, including Singapore.
Sewage surveillance data showed an increasing trend in the prevalence of XDV in Hong Kong. XDV is a JN.1-related variant.
The latest information does not suggest that XDV will cause a more severe disease than JN.1, XBB, and their descendant lineages.
The public (and visitors) are advised to maintain strict personal and environmental hygiene to protect themselves against infection and prevent the spread of the disease.
Additionally, public members are advised to note the latest recommendations on using COVID-19 vaccines in Hong Kong. High-risk priority groups are recommended to receive a dose of a COVID-19 vaccine at least six months since the last dose or infection, regardless of the number of doses received previously. For more details, please visit this CHP link.
Hong Kong is a special administrative region of China on the southern coast. Recent data indicates over 40 million people visited Hong Kong last year.
If you depart from the United States, the CDC writes, ' Make sure you are up-to-date on all routine and travel vaccines, such as chikungunya and typhoid, before visiting Hong Kong in May 2025.'
With over 16 million visitors in 2024, the Republic of Singapore has become a tourist favorite destination. However, before visiting this city-state in Southeast Asia in May 2025, it is highly recommended that you protect yourself from COVID-19.
Like most other countries, Singapore has experienced multiple waves of COVID-19 since the first confirmed case in January 2020.
According to the Ministry of Health (MOH) and the Communicable Diseases Agency, COVID-19 infections have significantly increased in the Spring of 2025.
The MOH estimated the number of COVID-19 cases from the last week of April to May 3, 2025, at 14,200, compared to 11,100 cases in the previous week.
The 28% increase in cases could be due to several factors, including waning population immunity.
Currently, LF.7 and NB.1.8, descendants of the JN.1 variant, are the main COVID-19 variants circulating in Singapore, accounting for more than two-thirds of locally sequenced cases.
JN.1 is also the variant used in formulating the current COVID-19 vaccines, which remain effective in protecting against severe illness.
Singapore's MOH wrote that 'individuals at increased risk of severe COVID-19, such as those aged 60 years and above, medically vulnerable individuals, or residents of aged care facilities, are recommended to keep updated with vaccinations, and receive an additional dose around one year after their last dose.
In the United States, the U.S. FDA recently approved Nuvaxovid™, a non-mRNA, JN.1 COVID-19 vaccine that is active against current circulating strains, including KP.2 and KP.3. This vaccine, and other travel vaccines, are offered at clinics and pharmacies in the U.S.



