Search API
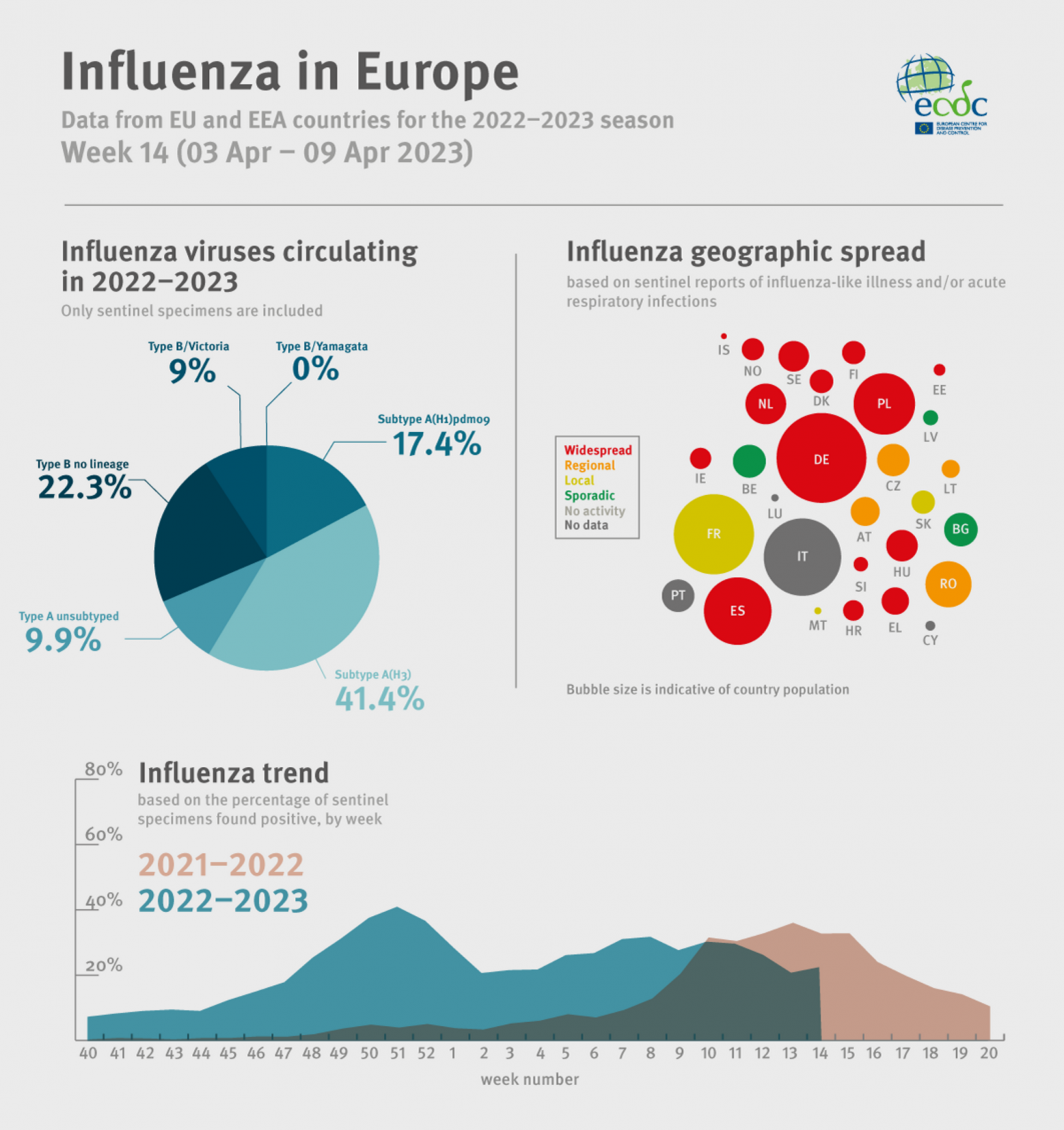
The European Centre for Disease Prevention and Control (ECDC) recently published an influenza update for week #14, indicating flu positivity was about 15%.
Announced on April 14, 2023, the ECDC confirmed of the 41 countries and areas reporting data on influenza viruses:
- 3 reported no activity (Georgia, Kazakhstan, and Kyrgyzstan),
- 9 reported sporadic spread (eastern, northern, and southern Region),
- 5 reported local spread (France, Malta, Serbia, Slovakia and Kosovo,
- 8 reported regional spread (Albania, Austria, Bosnia and Herzegovina, Czechia, Lithuania, Republic of Moldova, Romania and Ukraine, and,
- 16 reported widespread activity (across the Region).
The ECDC added seasonal influenza is a vaccine-preventable disease that annually infects over 10% of Europe's population.
When planning a European visit, the U.S. Centers for Disease Control and Prevention suggests discussing flu shot options with a healthcare provider.
SAB Biotherapeutics today announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track designation for SAB-176, an investigational therapeutic for Type A and Type B influenza illness in high-risk patients, including those who have anti-viral resistant strains.
SAB-176 offers the potential for additional treatment for influenza, particularly in higher-risk patients.
SAB also received FDA guidance and regulatory alignment on advancing SAB-176 into the next development phase by initiating a Phase 2b dose-range finding efficacy and safety trial in high-risk patients for developing severe disease.
SAB-176 is a novel, highly potent immunotherapy grounded in the fundamentals of the natural immune response to neutralize Type A and Type B influenza viruses, which mutate rapidly.
SAB-176 has undergone multiple clinical and pre-clinical studies, including a Phase 1 trial in healthy volunteers and a Phase 2a challenge study completed last year.
In the Phase 2a study, SAB-176 showed broad cross-protection that included influenza strains not explicitly targeted in manufacturing the therapeutic.
"We are pleased to receive the FDA Fast Track designation for SAB-176. Influenza continues to be one of the biggest public health challenges the world faces continuingly, with an excessively high number of hospitalizations and deaths each year," said Eddie Sullivan, Ph.D., co-founder, President & CEO of SAB Biotherapeutics, in a press release on April 13, 2023.
"We are excited about the potential role SAB-176 can play in tackling a highly mutagenic pathogen like influenza."
SAB-176 is also being studied in emerging and mutating pandemic strains by targeting multiple epitopes of the virus rather than a single epitope.
While Tamiflu® is an effective therapy for treating influenza if used within two days of symptom onset, some patients still develop severe disease and resistant strains of influenza to anti-viral drugs.
Throughout the 2022-2023 flu season in the U.S., over 171 million influenza vaccines were distributed, which remain available at health clinics and pharmacies.

The Florida Health Department reported as of week #13, there had been 59 travel-associated dengue cases. And, as of April 8, 2023, there are now 2 locally acquired dengue cases confirmed in 2023.
In 2022, Florida reported 903 travel-associated and 68 locally-acquired dengue cases.
In the Region of the Americas, 46 countries and territories reported dengue cases in 2022. For example, dengue was reported in 28 of 32 Mexico states last year.
These countries confirmed about 2.8 million dengue cases, representing a two-fold increase compared to 2021.
Dengue is a vaccine-preventable disease, and as of April 13, 2023, two vaccines are authorized in various countries.

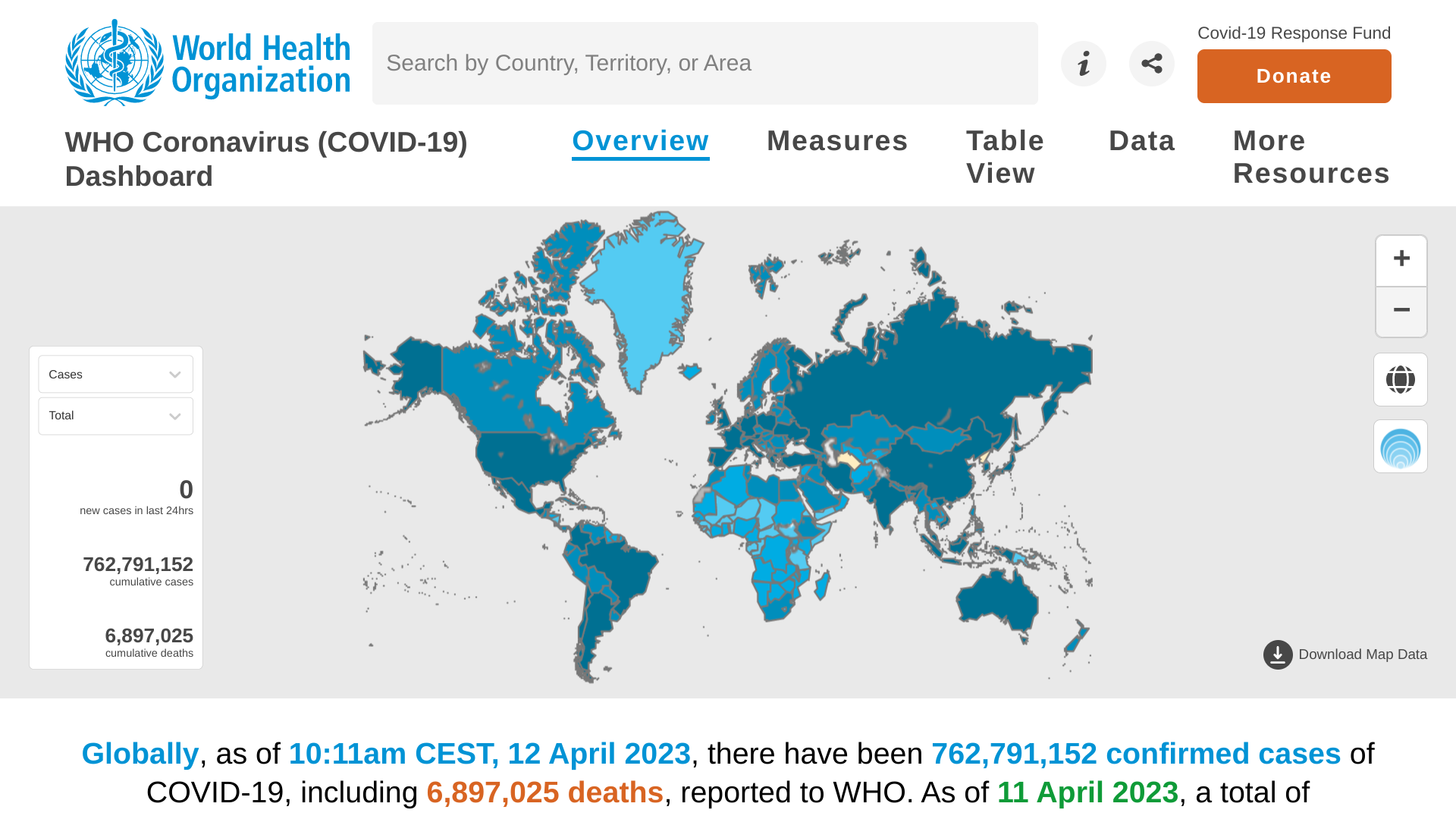
The World Health Organization (WHO) today published its weekly epidemiological update focused on the COVID-19 pandemic.
On April 13, 2023, the WHO's Edition #138 highlighted very positive trends.
Over the last 28 days (March 13 to April 9, 2023), COVID-19 cases decreased by 28%.
And related fatalities declined by 30% compared to the previous period.
However, contrary to the global trend, increases in reported COVID-19 cases and deaths were seen in the South-East Asia and Eastern Mediterranean regions and several individual countries.
At the regional level, the number of newly reported 28-day cases decreased across four of the six WHO regions: The African Region (-45%), the Western Pacific Region (-39%), the Region of the Americas (-33%), and the European Region (-22%).
While case numbers increased in two WHO regions: the South-East Asia Region (+481%) and the Eastern Mediterranean Region (+144%).
The highest numbers of new 28-day cases were reported at the country level from the U.S., the Russian Federation, the Republic of Korea, Brazil, and France.
The WHO suggests international travelers remain fully protected against COVID-19 by speaking with a healthcare provider to determine if a Spring or Summer COVId-19 booster is recommended, as well as various travel vaccines.
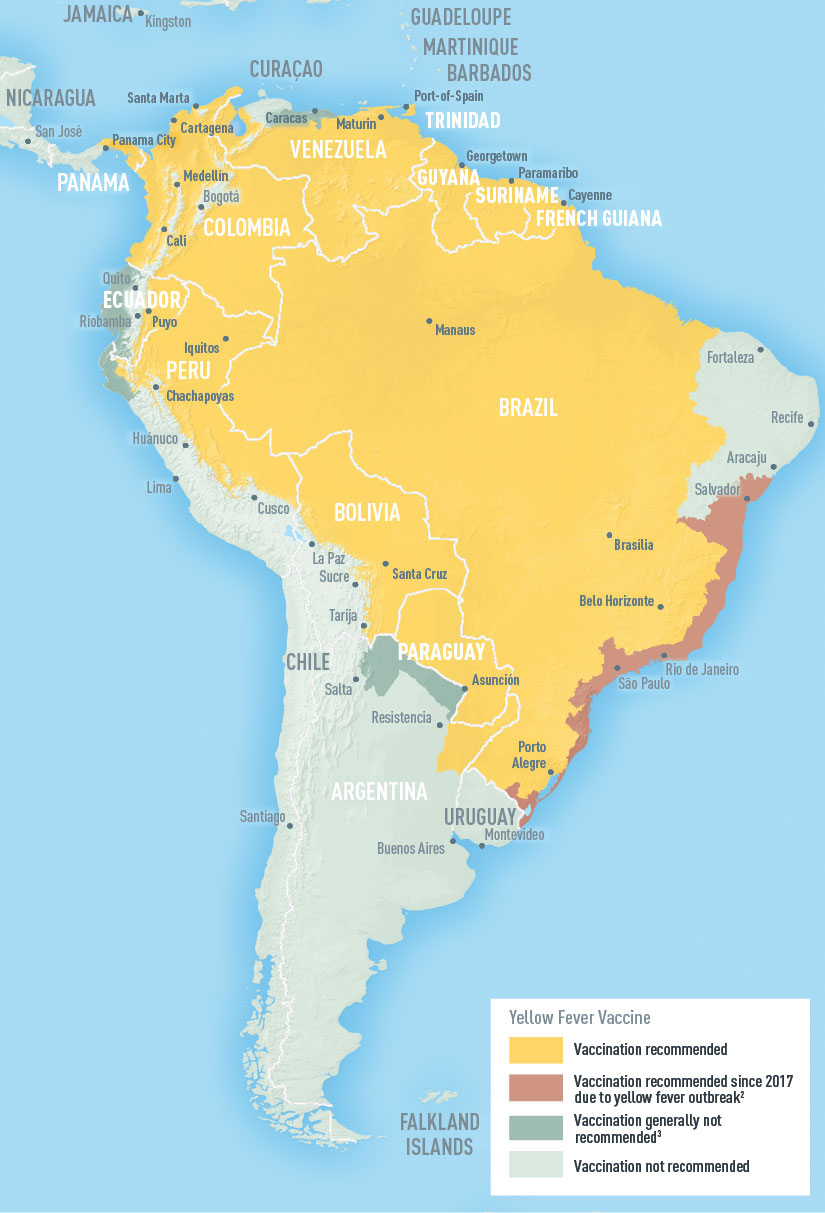
The yellow fever virus (YFV) is a reemerging global health threat in 2023, driven by several factors, including the increased spread of the mosquito vector.
Although protective YFV vaccines exist, recent outbreaks in South America indicate a void in treatment options in countries such as Brazil.
To establish innovative treatment options for patients with severe YFV infection, researchers recently tested 37 YFV-specific neutralizing monoclonal antibodies (mAbs) isolated from previously vaccinated humans.
They identified two capable of potently neutralizing multiple pathogenic primary YFV isolates.
Using hamster and nonhuman primate models of lethal YFV infection, they demonstrate that a single administration of either potently neutralizing mAbs during acute infection fully controlled viremia.
And it prevented severe disease and death in treated animals.
'Given the potential severity of YFV-induced disease, these results show that these antibodies could effectively save lives and fill a much-needed void in managing YFV cases during outbreaks, wrote these researchers in a Science Translational Medicines article published on March 29, 2023.
In the U.S., the YF-Vax® vaccine is available as of April 13, 2023, at certified clinics and travel pharmacies.
Furthermore, the International Certificate of Vaccination, known as the yellow card, is required to enter certain countries in 2023.
Various types of COVID-19 vaccines have been approved to reduce the disease burden during the recent pandemic. To better appreciate differences, South Korean researchers conducted an observational study to evaluate the effectiveness of Novavax's NVX-CoV2373 and BNT162b2 vaccines in protecting adults.
This non-peer-reviewed study was published on February 19, 2023, and compared the results from 3,019 recipients of NVX-CoV2373 and 3,027 recipients of BNT162b2 vaccines.
The 40-week risk ratios for recipients of the NVX-CoV2373 vaccine compared with recipients of the BNT162b2 vaccine were 1.169 (95% CI, 1.015 to 1.347) for laboratory-confirmed SARS-CoV-2 infection.
And 0.504 (95% CI, 0.126 to 2.014) for severe SARS-CoV-2 infection.
The estimated risk of severe infection was 0.001 events per 1000 persons (95% CI, 0 to 0.003) for the NVX-CoV2373 vaccine and 0.002 events per 1000 persons (95% CI, 0.001 to 0.006) for the BNT162b2 vaccine.
These researchers wrote this 'study identifies the reduced risk of SARS-CoV-2 infection and severe infection after receipt of three doses of either NVX-CoV2373 or BNT162b2 vaccines in Korean adults.
The study authors did not declare any competing industry interest.

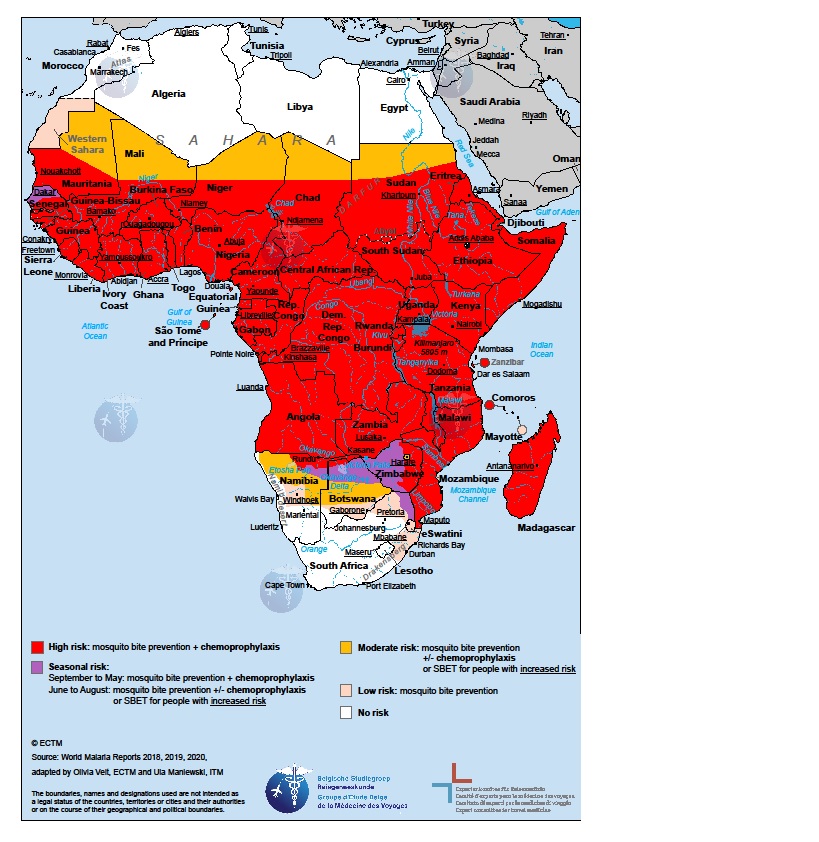
The University of Oxford today announced the R21/Matrix-M™ malaria vaccine was licensed for use in Ghana by the country’s Food and Drugs Authority.
This announcement marks the first regulatory clearance for the R21/Matrix-M malaria vaccine for use in any country for children at the highest risk of death from malaria.
According to the press release on April 13, 2023, the R21/Matrix-M vaccine has demonstrated high levels of efficacy and safety in Phase II trials, including amongst children who received a booster dose of R21/Matrix-M at one year following a primary three-dose regime.
The R21/Matrix-M malaria vaccine is a low-dose vaccine that can be manufactured at scale and modest cost, enabling as many as hundreds of millions of doses to be supplied to African countries which are suffering a significant malaria burden.
According to the U.S. Centers for Disease Control and Prevention, malaria is a vaccine-preventable mosquito-borne disease caused by a parasite. Malaria vaccines like Mosquirix™ and R21 have been reported effective at preventing disease.
As of April 13, 2023, these malaria vaccines are unavailable in the U.S.
The U.S. government today announced it is releasing the National COVID-19 Preparedness Plan. This plan lays out the roadmap to help fight COVID-19 in the future.
'We look to a future when Americans no longer fear lockdowns, shutdowns, and our kids not going to school,' wrote the U.S. government on April 12, 2023.
'It's a future when the country relies on the powerful layers of protection we have built."
"And invests in the next generation of tools to stay ahead of this coronavirus."
The President's National COVID-19 Preparedness Plan focuses on four key goals, which are linked here.