Search API
Valneva SE today announced positive final results regarding the immunogenicity and safety from its Phase 2 study, VLA15-221, of the Lyme disease vaccine candidate, VLA15.
According to Valneva's press release on November 26, 2025, this study's findings demonstrated a strong anamnestic immune response and a favorable safety profile six months after the third booster dose (at month 48) across all age groups.
Furthermore, these results confirm that the vaccine is compatible with the expected benefits of annual vaccination before the start of each Lyme season, which coincides with increased outdoor activities in the Northeast and Midwest regions of the United States.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release. "Lyme disease continues to expand geographically and remains a pressing unmet medical need affecting communities across the Northern Hemisphere."
"Each set of positive results moves us closer to the possibility of making this vaccine available to adults, adolescents, and children living in Lyme-endemic areas."
This is very encouraging news as there are currently no approved human vaccines for Lyme disease, and VLA15 has advanced the furthest in clinical development.
Valneva partnered with Pfizer Inc. in April 2020 to develop and commercialize VLA15.
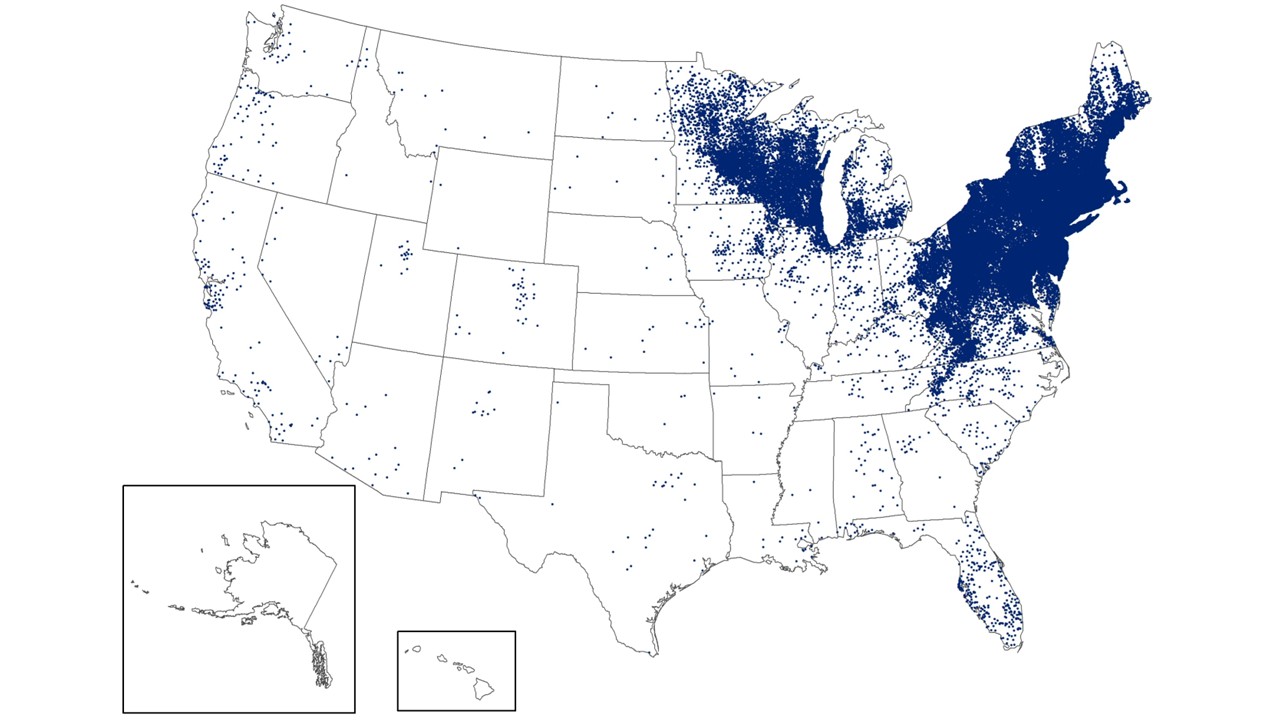
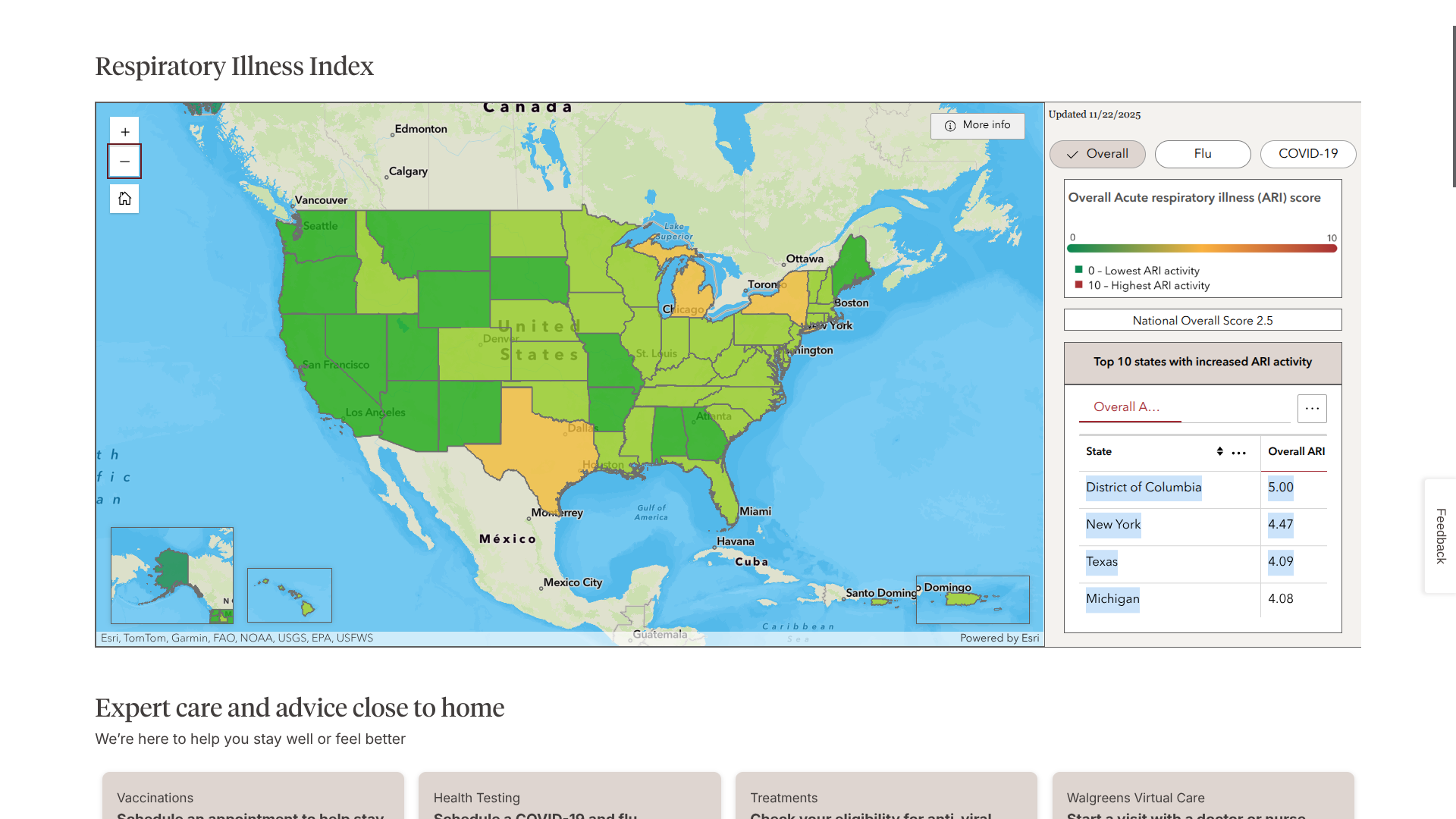
Launched in early November 2025, the Walgreens Acute Respiratory Index (ARI) for 2025-2026 tracks influenza and COVID-19 hotspots using prescription, testing, and over-the-counter product purchase data from its locations nationwide.
As of November 22, 2025, the District of Columbia, New York, Texas, and Michigan lead Walgreens' ARI index.
Nationwide, the overall ARI score was 2.5 out of 5.0.
Walgreens' unique digital tool offers a more comprehensive view of respiratory illness trends across the U.S., enabling the public, healthcare providers, and health officials to make more informed decisions throughout the respiratory virus season.
Rick Gates, Walgreens' chief pharmacy officer, commented in a press release, "Vaccination remains the safest and most effective way to prevent highly contagious illnesses like flu, RSV, and COVID-19."
Previously, the U.S. CDC FluView for Week 47 will be posted on November 21, 2025, stating 'seasonal influenza activity in the United States remains low nationally but is increasing.'
Various flu shots are offered throughout the influenza season at most community pharmacies.
On the occasion of the Jubilee of the Poor, a special holy year which occurs once every 25 years, the European Centre for Disease Prevention and Control (ECDC) has been conducting enhanced monitoring through its epidemic intelligence activities.
With multiple events that take place throughout the year and nning until December 2025, it is estimated that more than 35 million pilgrims will visit Rome during the Jubilee of the Poor.
As of November 21, 2025, the ECDC says the probability of EU/EEA citizens becoming infected with communicable diseases during the Jubilee 2025 is low if general preventive measures are followed, such as influenza vaccination according to national immunisation schedules.

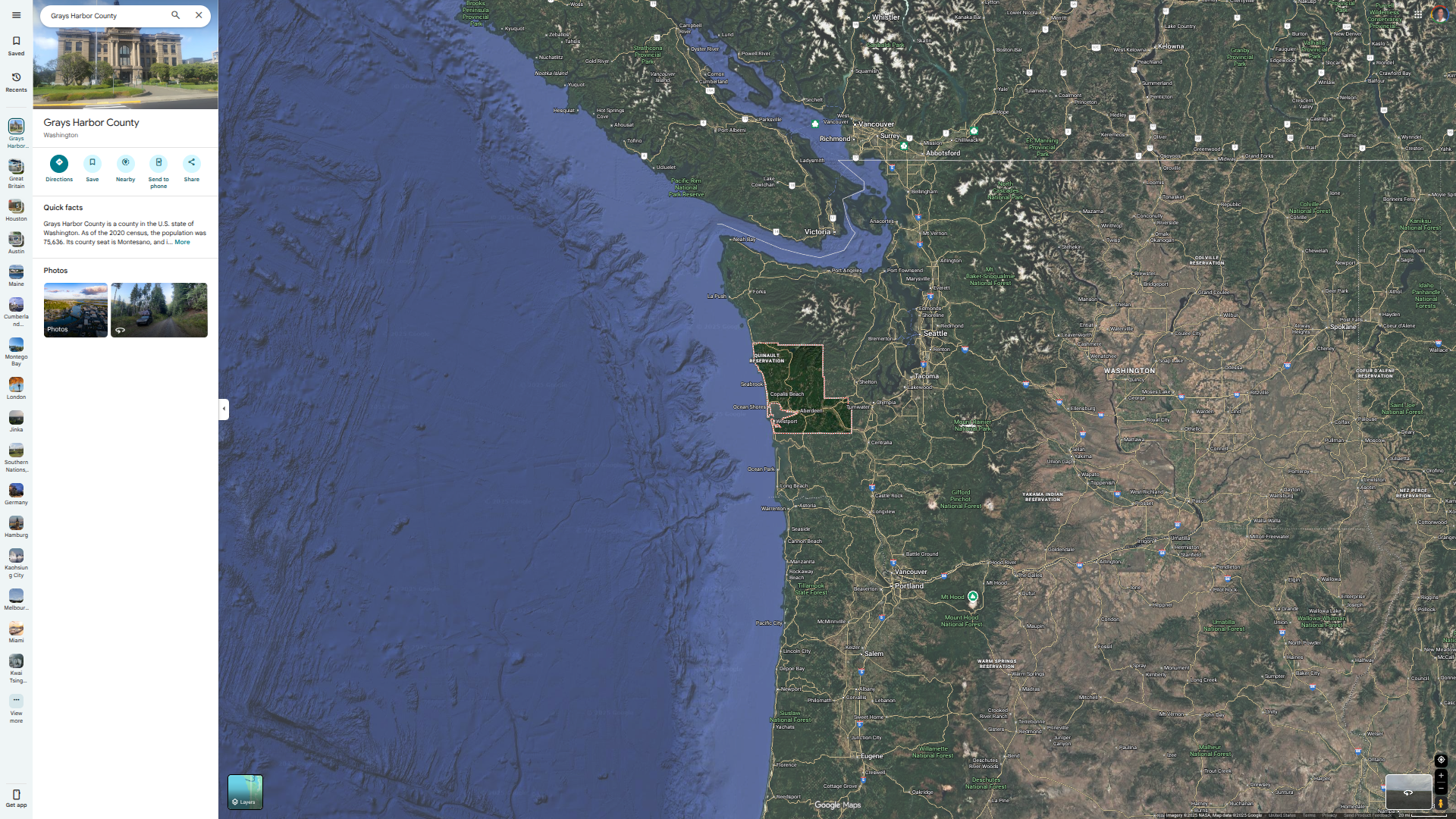
The Washington State Department of Health (WDOH) recently confirmed the death of an older adult from Grays Harbor County related to a specific type of 'bird flu.' The individual had underlying health conditions and was receiving treatment for an infection of the H5N5 avian influenza virus.
This report from WDOH, issued on November 21, 2025, marks the first human case of avian influenza A (H5N5) reported worldwide.
Washington Governor Bob Ferguson stated in a Facebook post, "My condolences to this individual's family and loved ones. Thank you to the dedicated medical professionals who cared for them, and to the public health experts working to monitor the situation and educate the public."
This person had a backyard flock of mixed domestic birds. DOH sampling identified avian influenza virus in the environment of the flock, making exposure to the domestic poultry, their environment, or wild birds the most likely source of exposure for this patient. People who had exposure to the backyard flock and its environment are also being monitored by local health authorities for symptoms.
The WDOG says the risk to the public remains low as no other people involved have tested positive for avian influenza.
Public health officials will continue to monitor anyone who was in close contact with the patient for symptoms to ensure that human-to-human spread has not occurred. There is no evidence of transmission of this virus between people.
From a prevention perspective, at least 20 H5 influenza vaccines are licensed worldwide.
As of November 25, 2025, U.S. FDA-approved avian influenza vaccines are not commercially available in the USA.
The Pan American Health Organization (PAHO) recently announced that the entire Region of the Americas has lost its verification of endemic measles transmission elimination due to ongoing outbreaks in several countries.
For example, in early November, Canada lost its measles elimination status.
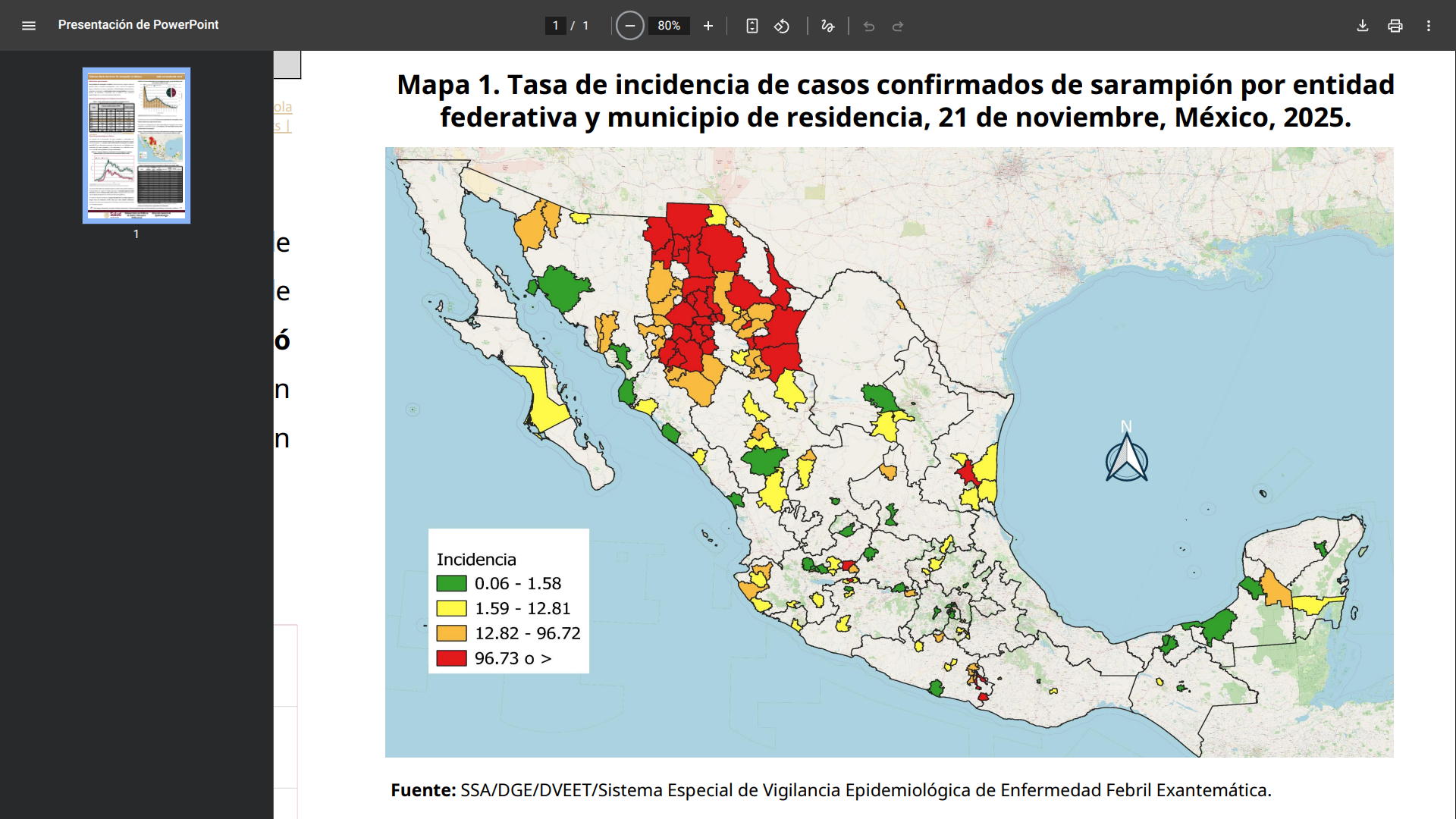
As of November 24, 2023, Mexico's Ministry of Health reported approximately 5,300 cumulative confirmed measles cases this year, primarily in the northern region, just south of New Mexico and Texas.
This data is comparable to Canada's, indicating that Mexico may also lose its measles-free status.
Meanwhile, the United States, located between Canada and Mexico, has reported around 1,753 measles cases this year, with a few ongoing outbreaks, particularly in northern South Carolina.
The standard recommendation shared among these countries and states is that most people should be fully vaccinated with the MMR vaccine to protect themselves from the easily transmissible virus.
The Maine CDC recently announced it was investigating a cluster of seven cases of shigellosis among residents of Cumberland, Kennebec, and Androscoggin counties. So far in 2025, there have been 22 cases, preliminary data as of November 19, 2025.
The Maine CDC says that in the past five years, the median number of annual shigellosis cases was 11.
Shigellosis is an acute enteric infection transmitted through the fecal-oral route, directly through person-to-person contact, including sexual contact, and indirectly through contaminated food, water, and other routes.
The World Health Organization says it is evaluating the role of vaccines against Shigella on antibiotic use. Although several candidate Shigella vaccines are being evaluated at different stages of preclinical and clinical development, currently, no licensed vaccines against Shigella diarrhoea are widely available.
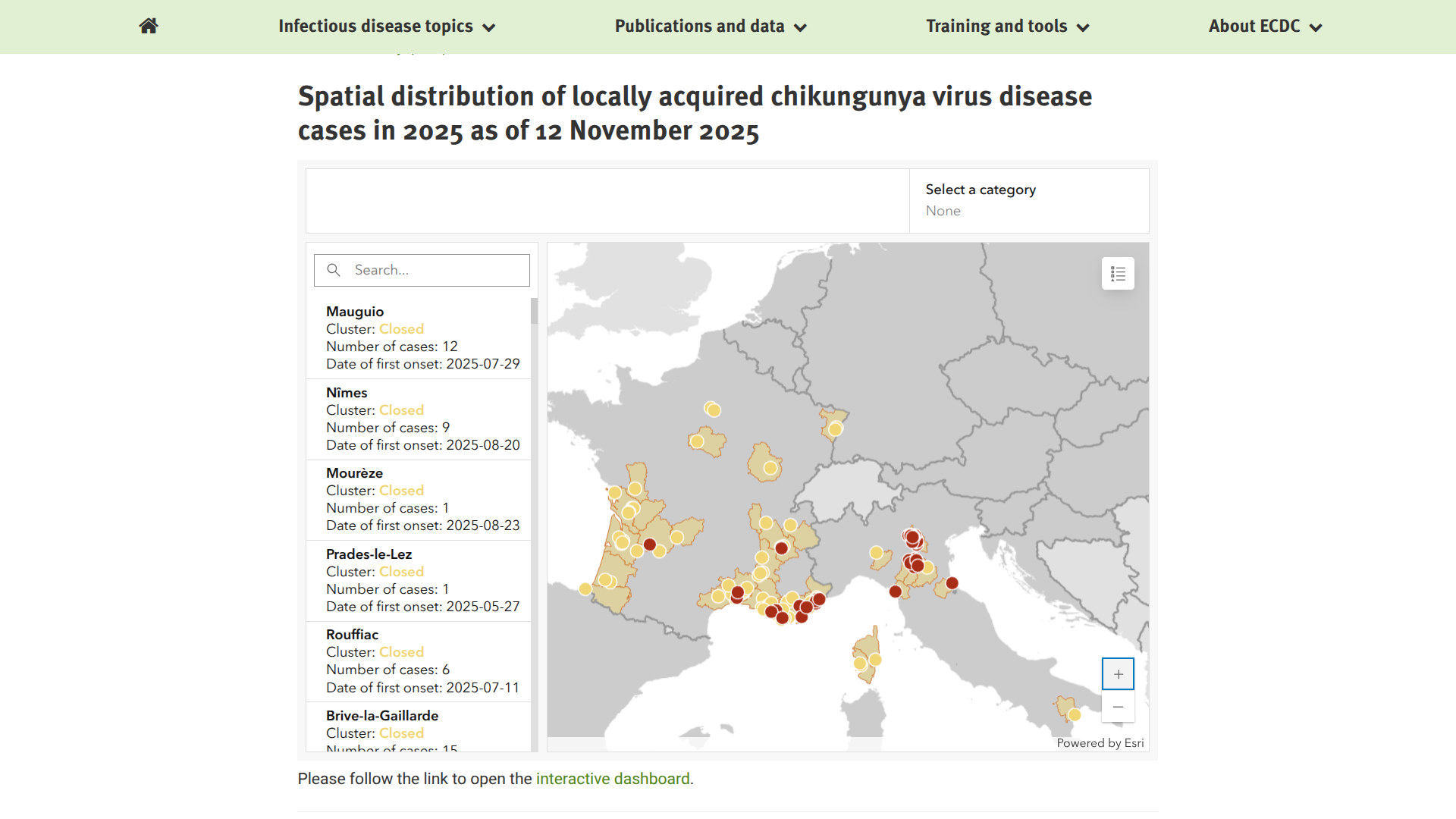
The French health ministry today published an updated Bulletin summarizing the unusually high number of chikungunya virus cases this year.
As of November 20, 2025, there have been 87 locally acquired cases of chikungunya in France: 43 in the Gard and 44 in the Hérault, located along France's Mediterranean coast.
Additionally, France confirmed 1,052 imported cases, with about 72% related to travelers from the department of La Réunion, located in the southwest Indian Ocean.
Seperately, Italy has reported 384 local cases, with the largest cluster located in Carpi, San Prospero, Soliera, Novellara, Cavezzo, Modena, Nonantola, Correggio, Novi di Modena, and Cesenatico.
The reasons behind these outbreaks may be weather-related.
On November 17, 2025, the European CDC reported that, according to data in the scientific literature, the ambient temperature is one of the most important environmental factors influencing the mosquito-borne transmission of the chikungunya virus.
The optimal daily average temperature for chikungunya transmission by Ae. albopictus in temperate zones of the Northern Hemisphere is 24–26 °C.
However, transmission might occur within the temperature range of 12 – 30 °C.
Currently, the U.S. CDC advises travelers to these areas to speak with a healthcare provider about chikungunya vaccination options.