Search API
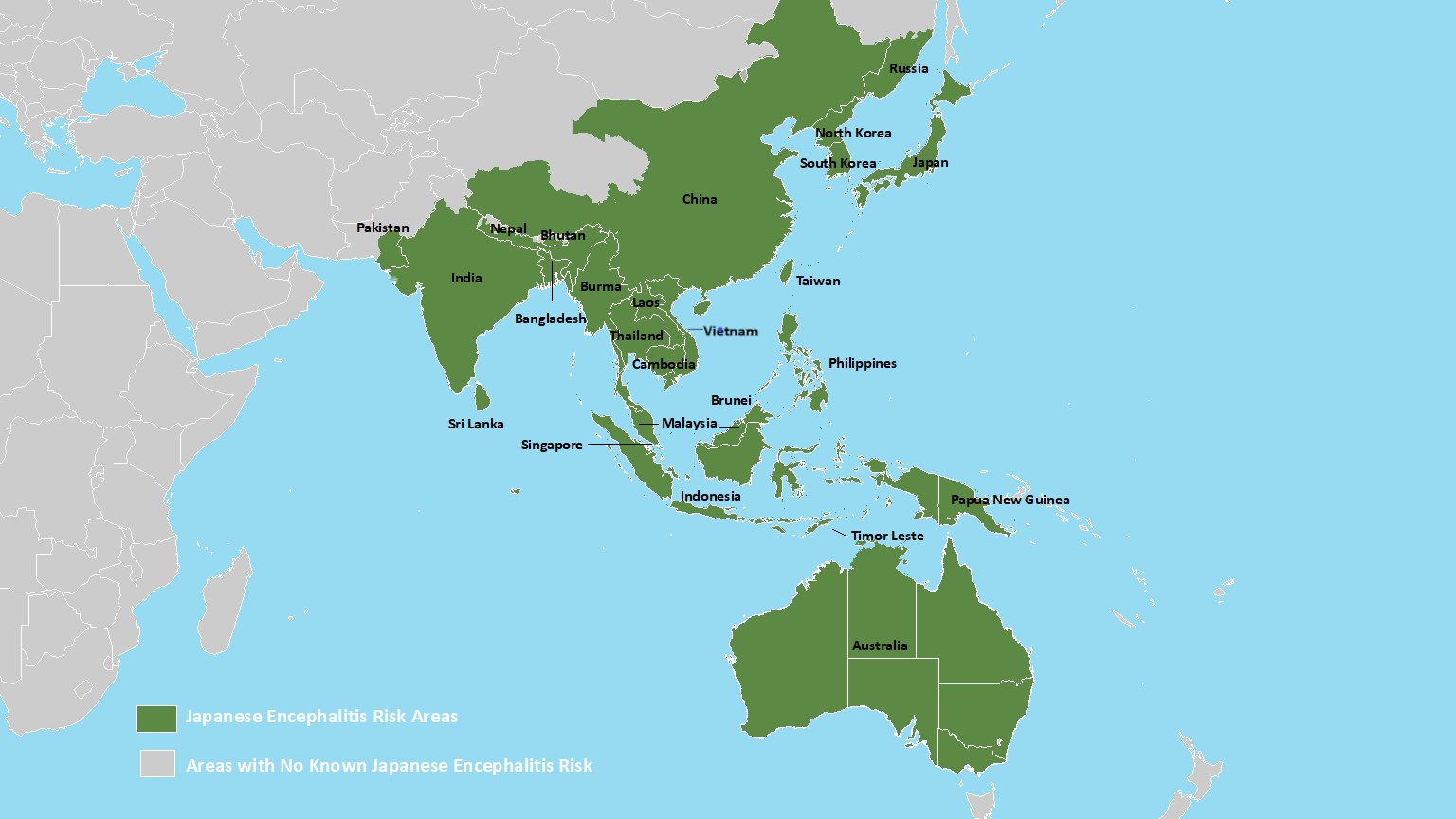
Japanese encephalitis virus (JEV) is the leading cause of epidemic encephalitis worldwide and is prevalent across Asia and the Pacific.
A recent study published in the journal Nature highlights significant public health concerns stemming from changes in the dominant genotype, the emergence of epidemics in new regions, and the re-emergence of previously dormant genotypes.
The re-emergence of specific genotypes in Indonesia after 37 years, coupled with JEV-related fatalities in Australia, Nepal, and Taiwan, highlights the need for critical control measures.
Nepal's Department of Health Services has confirmed 164 cases of Japanese encephalitis in 2025, compared to 86 last year.
In 2024, 23 people died in Nepal infected with the JEV.
Similarly, the resurgence of genotype in China after 57 years and its circulation in Korea underscore the need for continuous surveillance and proactive vaccinations.
According to the U.S. CDC, a Japanese encephalitis vaccine (IXIARO) is available in the United States, approved for use in children aged 2 months and older and adults.
The CDC says this approved vaccine should be considered for some travelers before visiting high-risk areas in 2025.
In the United States, IXIARO is commercailly offered at travel clinics and pharmacies.
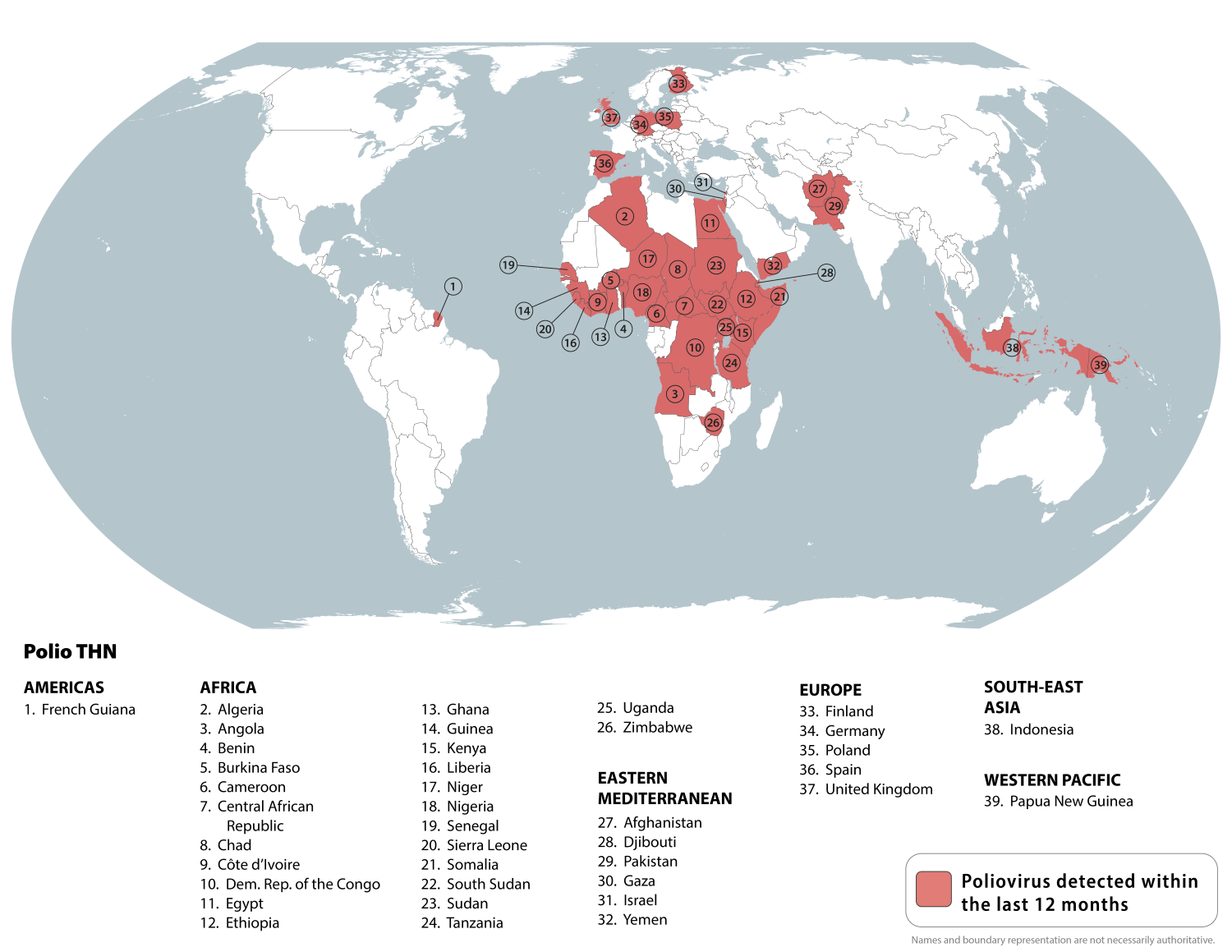
According to the Global Polio Eradication Initiative (GPEI), 2 billion doses of the novel oral polio vaccine type 2 (nOPV2) have now been administered to children around the world, primarily in Africa.
Since the WHO approved its use in 2020, the nOPV2 vaccine has been an essential part of intensified efforts to curb outbreaks of circulating type 2 variant poliovirus (cVDPV2) in 35 countries. It has been 'triple-locked' through genetic engineering to prevent it from becoming harmful or producing mutations, says the GPEI.
The U.S. CDC Advisory Committee on Immunization Practices presentations on February 28, 2024, included an Introduction and Considerations for the Potential Use of nOPV2 as an Outbreak Control Measure in the U.S.
The CDC currently publishes a Level 2 Travel Health Notice regarding the risk of polio in 39 countries.
As of November 10, 2025, the IPV polio vaccine is offered in the United States, and booster doses are recommended for specific international travelers.
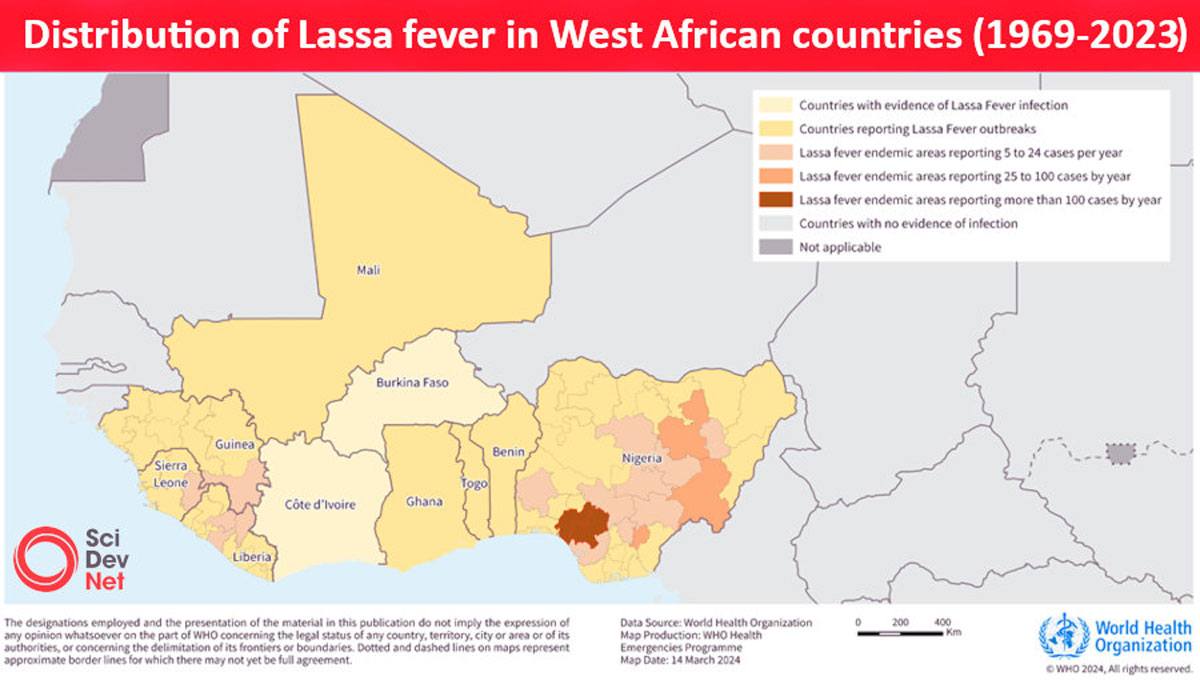
Currently, no vaccines or therapeutics are licensed against Lassa fever, an acute viral hemorrhagic illness caused by Lassa virus (LASV) that is responsible for thousands of deaths each year across West Africa, where the disease is endemic.
The findings from a first-in-human, Phase 1 clinical trial of IAVI's LASV vaccine candidate, published in the New England Journal of Medicine on November 6, 2025, demonstrate that one dose of the vaccine elicits robust and long-lasting immune responses and has an acceptable safety profile.
"Lassa fever is a cruel disease which has plagued West Africa for decades, including a deadly outbreak in Nigeria this year," said Dr. Kent Kester, Executive Director of Vaccine R&D at CEPI, in a press release.
"The promising Phase 1 data for IAVI's vaccine candidate takes us one step closer towards a much-needed Lassa fever vaccine, which, if successful, could save thousands of lives and avert millions of dollars of societal costs in the West African countries that bear the burden of this disease."
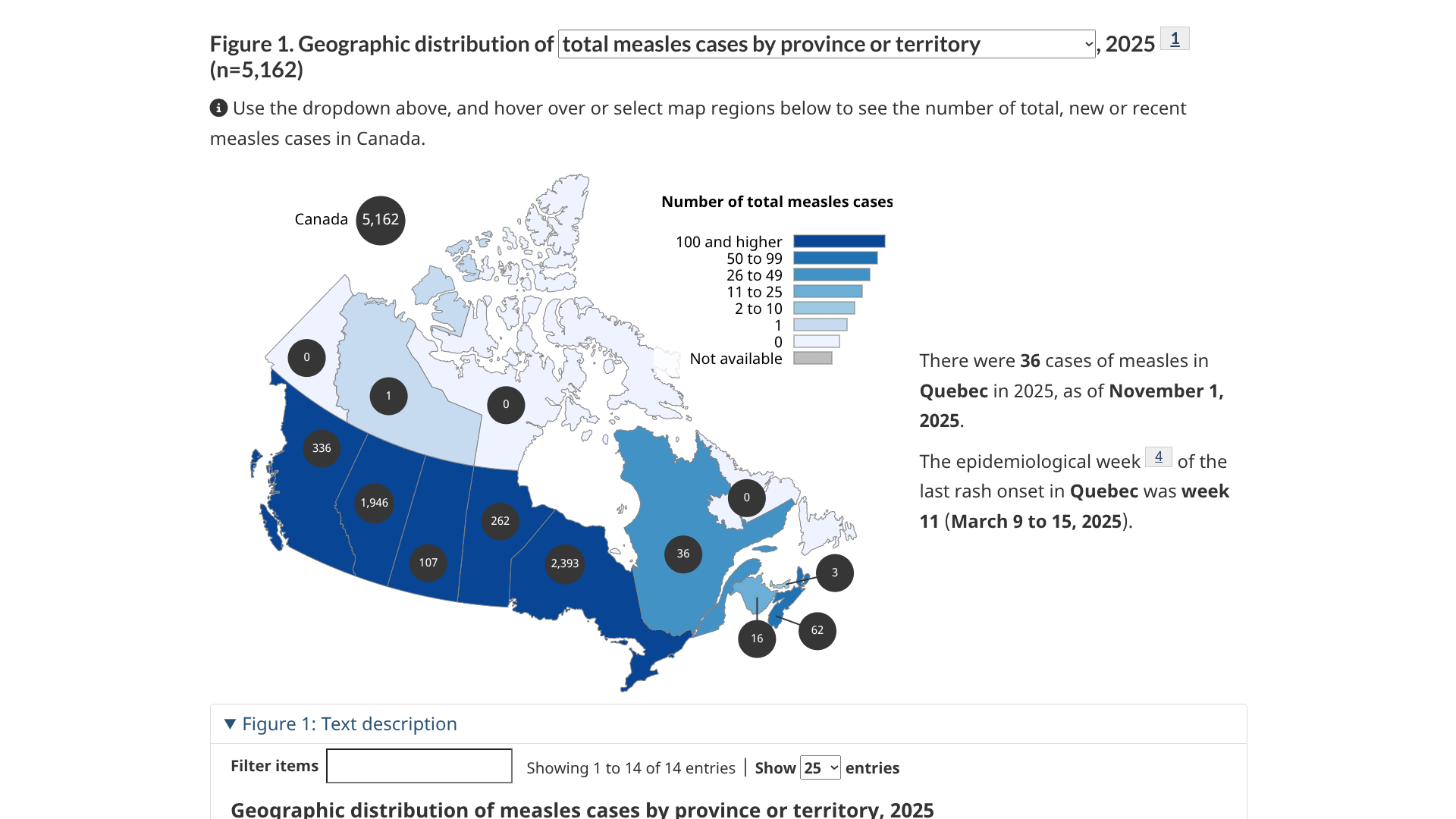
The Pan American Health Organization (PAHO) today announced Canada has lost its measles elimination status, which was attained in 1998.
According to the PAHO press release on November 10, 2025, Canada can re-establish its measles status once transmission of the measles virus has been interrupted for at least 12 months.
During 2025, over 80% of Canada's 5,162 measles cases were reported in Ontario and Alberta.
Currently, the U.S. CDC advises all people traveling to Canada to ensure they are fully vaccinated against measles.
The Israeli Ministry of Health recently reported the ninth measles-related fatality in a child since an outbreak began earlier in 2025.
On November 9, 2025, a child from Jerusalem with an underlying health condition, who had received one dose of the measles vaccine, passed away at an emergency department due to complications from the disease.
As of today, 16 patients are hospitalized after contracting measles.
Until now, all previous measles cases involved healthy infants with no underlying conditions who were unvaccinated.
The Ministry of Health reiterated in a press release that measles is preventable with a safe and effective vaccine. It emphasizes the importance of seeking medical care promptly if symptoms appear or if exposure to measles is suspected.
Since May 2025, the U.S. CDC has included Israel in its Global Measles travel advisory.
The CDC says all international travelers should be fully vaccinated against measles before departing abroad. Measles vaccination services are offered at travel clinics and pharmacies in 2025.
An extensive real-world study published in the Journal of Infection suggests that the long-acting monoclonal antibody nirsevimab (Beyfortus) provides protection against respiratory syncytial virus (RSV) infection for children younger than 2 years for up to one year.
Published on November 6, 2025, this study found RSV infection was confirmed in 8.5%, 8.0%, and 20.7% of children who received nirsevimab within 6 months, from 6 to 11 months, and beyond 12 months before RSV testing, respectively, while 16.6%, 17.7%, and 17.3% of those who didn't receive nirsevimab were infected in each corresponding period.
These researchers wrote that the results of this study may stimulate discussions regarding repeated dosing schedules for infants and young children.
Grants from the Japan Agency for Medical Research and Development and others supported this research.
Nirsevimab has been approved and is used in several countries, including the United States, to prevent RSV infections and their associated severe outcomes.

The spread of H5N1 influenza (bird flu) in animals with spillover into human populations remains a global health risk.
To address this serious issue, various vaccines have been developed over the past few years.
However, researchers at the University of Maryland School of Medicine's Center for Vaccine Development and Global Health reported encouraging results yesterday from an innovative early-phase clinical trial that found an experimental intranasal vaccine triggered a broad immune response against multiple strains of H5N1.
The study, funded by a grant from the National Institutes of Allergy and Infectious Diseases, was published in the journal Nature Communications on November 6, 2025, and highlights the potential of mucosal immunization strategies — where vaccines are squirted into the nostrils — to prime immune defenses against diverse influenza strains.
The NanoVax H5 intranasal vaccine was found to be safe and well-tolerated. Notably, only people who received the boosted nasal vaccine showed strong immune "priming"—meaning their immune systems were activated and ready to respond—as revealed later, when they were given a single dose of an intramuscular H5 flu shot.
Even on its own without a booster, the NanoVax H5 intranasal vaccine triggered mucosal and systemic immune defenses —something other intranasal recombinant H5 flu vaccines have not achieved in clinical trials.
"The vaccine also helped the immune system recognize multiple versions of the H5N1 virus, which is key because there are different versions of the virus and they change over time," said study co-lead author Meagan E. Deming, MD, PhD, Assistant Professor of Medicine at UMSOM, in a press release.
"The use of the adjuvant also suggests this approach might allow for lower doses of the vaccine, which could make our current vaccine stocks available to more people in the event of an outbreak."
IN February 2025, the U.S. Secretary of Agriculture announced a $1 billion comprehensive strategy to curb highly pathogenic avian influenza outbreaks and protect the U.S. poultry industry, and support vaccine development efforts.

When the U.S. Centers for Disease Control and Prevention (CDC) issued a Level 1 - Practice Usual Precautions notice in September 2025, it highlighted that the bites of infected midges and mosquitoes are spreading the Oropouche virus in Cuba.
As of November 7, 2025, a total of 4,119 locally acquired Oropouche cases have been reported in Cuba this year.
In South America, Brazil leads the Americas with about 12,000 cases.
Last year, 103 cases of Oropouche virus were reported among individuals in Florida who had traveled to endemic areas, such as Brazil and Cuba.
Currently, no vaccines are available to prevent Oropouche disease, says the CDC.

The Canadian government recently reissued its travel guidance for visitors to the Republic of Costa Rica. As of October 28, 2025, Canada advises a high caution level due to widespread crime in Costa Rica's urban centers and coastal spots.
As of November 5, 2025, this advisory does not ban travel but urges awareness. In San José, the Canadian Embassy provides support to Canadians in Costa Rica.
According to the Tico Times, Costa Rica attracts thousands of Canadians every year. During the early part of 2025, over 143,000 people visited.
From a health perspective, the U.S. CDC has included Costa Rica in several notices, including the northern expansion of the New World screwworm, which had previously been eliminated in Costa Rica.
Additionally, Chikungunya, Dengue, Malaria, Measles, and Zika virus infections have been reported this year, with rates varying by location in this Central American country.