Search API
Moderna, Inc. today announced that the first participant in the U.S. has been dosed in the Nova 301 Trial, a pivotal Phase 3 randomized clinical trial evaluating the efficacy, safety, and immunogenicity of an investigational norovirus vaccine, mRNA-1403.
As of October 1, 2024, no U.S. FDA-approved norovirus vaccines are available.
"Norovirus is a significant public health concern that affects millions of people worldwide each year, leading to severe symptoms and, in some cases, hospitalization," said Stéphane Bancel, CEO of Moderna, in a press release.
Enteric viruses, including norovirus, are a leading cause of diarrheal diseases, resulting in significant morbidity and mortality worldwide, particularly among young children and older adults.
Norovirus is highly contagious and a leading cause of diarrheal disease globally, associated with 18% of all AGE, resulting in approximately 200,000 deaths per year and substantial healthcare costs.
The Nova 301 Phase 3 trial is a randomized, observer-blind, placebo-controlled trial evaluating the efficacy, safety, and immunogenicity of mRNA-1403. The trial aims to enroll approximately 25,000 participants 18 years of age and older globally, including in countries in the Northern Hemisphere (U.S., Canada, UK, Japan), the equatorial region, and the Southern Hemisphere (Australia and countries in South America).
Approximately 20,000 participants 60 years of age and older and 5,000 participants between 18 and 59 years of age will be enrolled to assess the investigational vaccine's ability to protect against moderate to severe norovirus acute gastroenteritis (AGE) in adults, with a focus on the older age group that is at the highest risk of severe outcomes, including hospitalization.

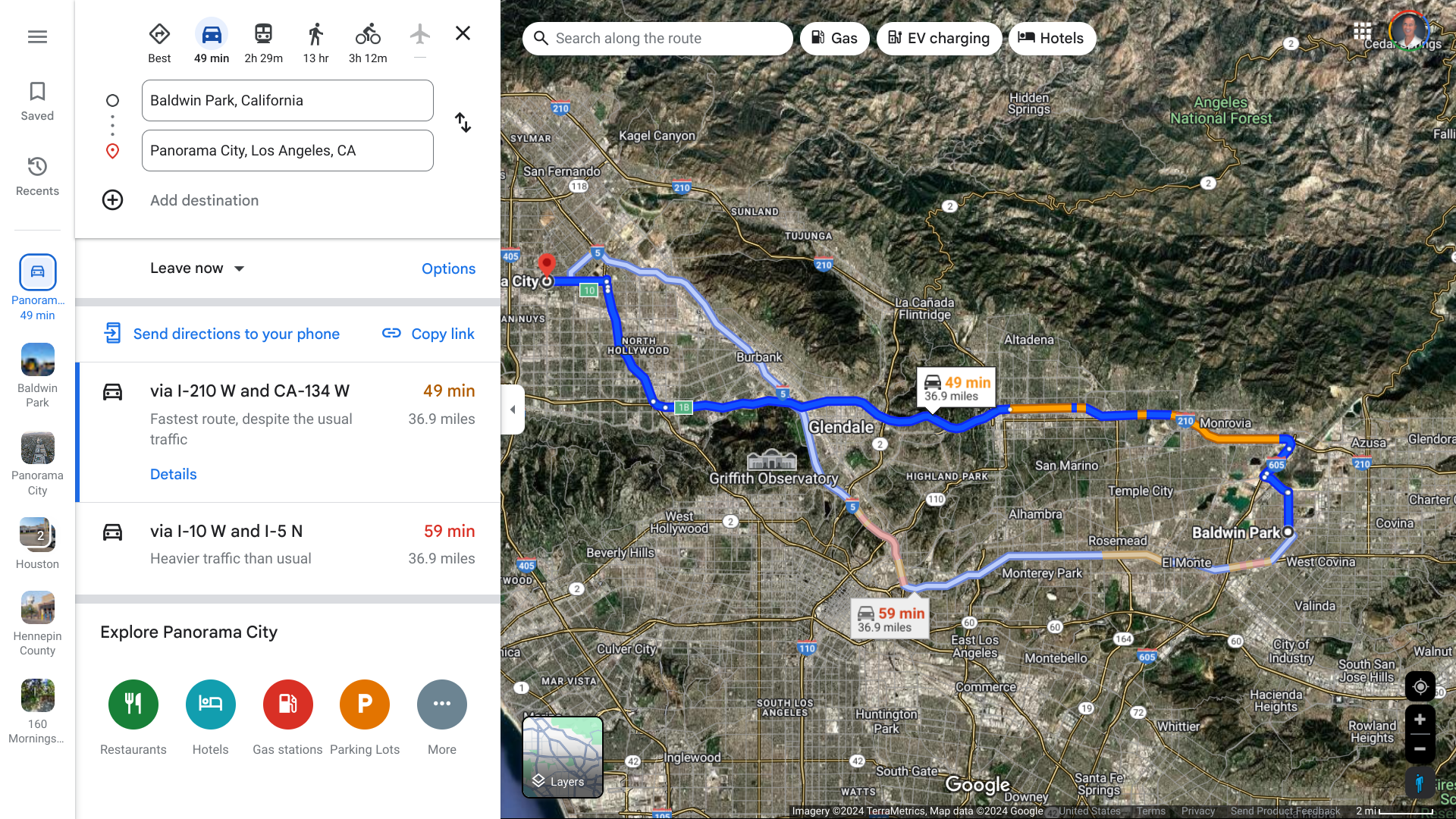
The Los Angeles County Department of Public Health is investigating another case of locally acquired Dengue in a Panorama City resident. The person had no history of travel to areas where Dengue is endemic.
This case of local transmission is not related to the cluster of 3 cases in the City of Baldwin Park on September 18, 2024.
About 55 miles are separating these cities.
Long Beach and Pasadena previously confirmed cases of locally acquired Dengue in fall 2023.
Although Aedes mosquitoes are common in LA County and can spread the dengue virus, almost all previously reported dengue cases in LA County have been associated with travel to a country where Dengue is endemic.
“We are seeing the local dengue transmission unprecedented in Los Angeles County. Preventing mosquito bites and mosquito breeding is the best way to stop the ongoing local transmission of Dengue,” said Muntu Davis, MD, MPH, Los Angeles County Health Officer, in a press release.
As of September 30, 2024, Public Health has reported four cases of locally acquired Dengue. These local transmission cases are extremely rare in LA County, and residents are urged to take proactive steps to prevent mosquito breeding and bites.
The U.S. CDC reported that 50 jurisdictions, led by Florida, New Jersey, New York, and Puerto Rico, had reported over 5,300 dengue cases this year.
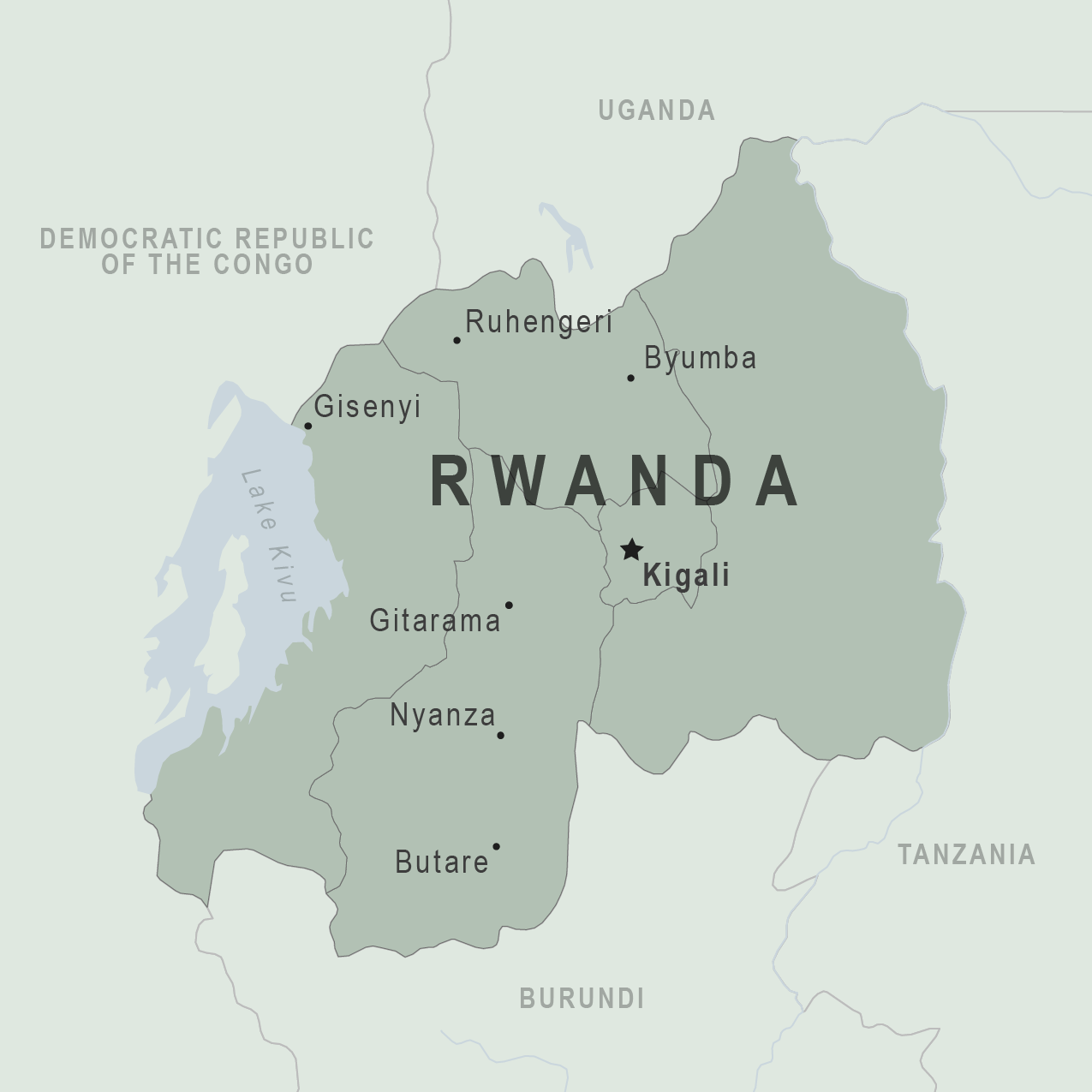
With eight fatalities and numerous infections reported, the Republic of Rwanda's Marburg virus disease (MVD) outbreak has raised global alarms as the origin of these infections has yet to be determined.
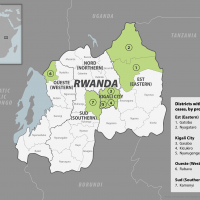
Since September 29, 2024, Rwandan health authorities have reported 26 confirmed cases in seven of the country's 30 districts.
Additionally, 161 people who came into contact with the reported cases have been identified and are being monitored. Staff at two hospitals in Kigali, home to about 1.7 million residents, are also being evaluated.
In support of the ongoing efforts, the World Health Organization (WHO) is mobilizing expertise, outbreak response tools, and emergency medical supplies to reinforce the control measures rolled out to curb the virus.
The WHO says illness caused by Marburg infections begins abruptly, with high fever, severe headache, and severe malaise. Many patients develop severe hemorrhagic symptoms within seven days. The virus is transmitted to people from fruit bats and spreads among humans through direct contact with the bodily fluids of infected people, surfaces, and materials.
"We're rapidly setting all the critical outbreak response aspects in motion to support Rwanda halt the spread of this virus swiftly and effectively," said Dr. Matshidiso Moeti, WHO Regional Director for Africa, in a press release on September 28, 2024.
WHO is also coordinating efforts to reinforce collaborative cross-border measures for readiness and response in countries neighboring Rwanda to ensure timely detection and control of the virus to avert further spread.
As of September 30, 2024, the WHO has not issued a travel alert regarding this Marburg outbreak. However, Rwanda is included in the clade I mpox outbreak in Africa.
Since 1967, when MVD was first recognized in a German lab spillover event, countries such as DR Congo, Equatorial Guinea, Cameroon, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, Tanzania, Yugoslavia, Uganda, and Rwanda have confirmed cases.
The WHO is coordinating a consortium of experts to promote the preclinical and clinical development of vaccines and therapeutics against MVD.
In March 2022, the WHO R&D Blueprint team defined the Strategic Agenda for Filovirus Research and Monitoring to establish research priorities for developing vaccines targeting filovirus diseases during the next decade.
While no product has been approved yet, one innovative Marburg vaccine candidate has progressed in 2024.
Public Health Vaccines, LLC launched its Phase 1 clinical trial (NCT06265012) in March 2024 to evaluate the safety and immunogenicity of its single-dose PHV01 (rVSV∆G-MARV-GP [Angola]) vaccine. The PHV01 vaccine is leveraging the proven recombinant vesicular stomatitis virus vector platform initially developed by the Public Health Agency of Canada.
The U.S. Biomedical Advanced Research and Development Authority (BARDA) has funded this vaccine research. If PHV01's development continues to succeed, BARDA has the option to provide up to $72 million in funding to continue development through Phase 2 clinical testing.
Update: As of September 30, 2024, Rwanda reported its 9th Marburg-related fatality.
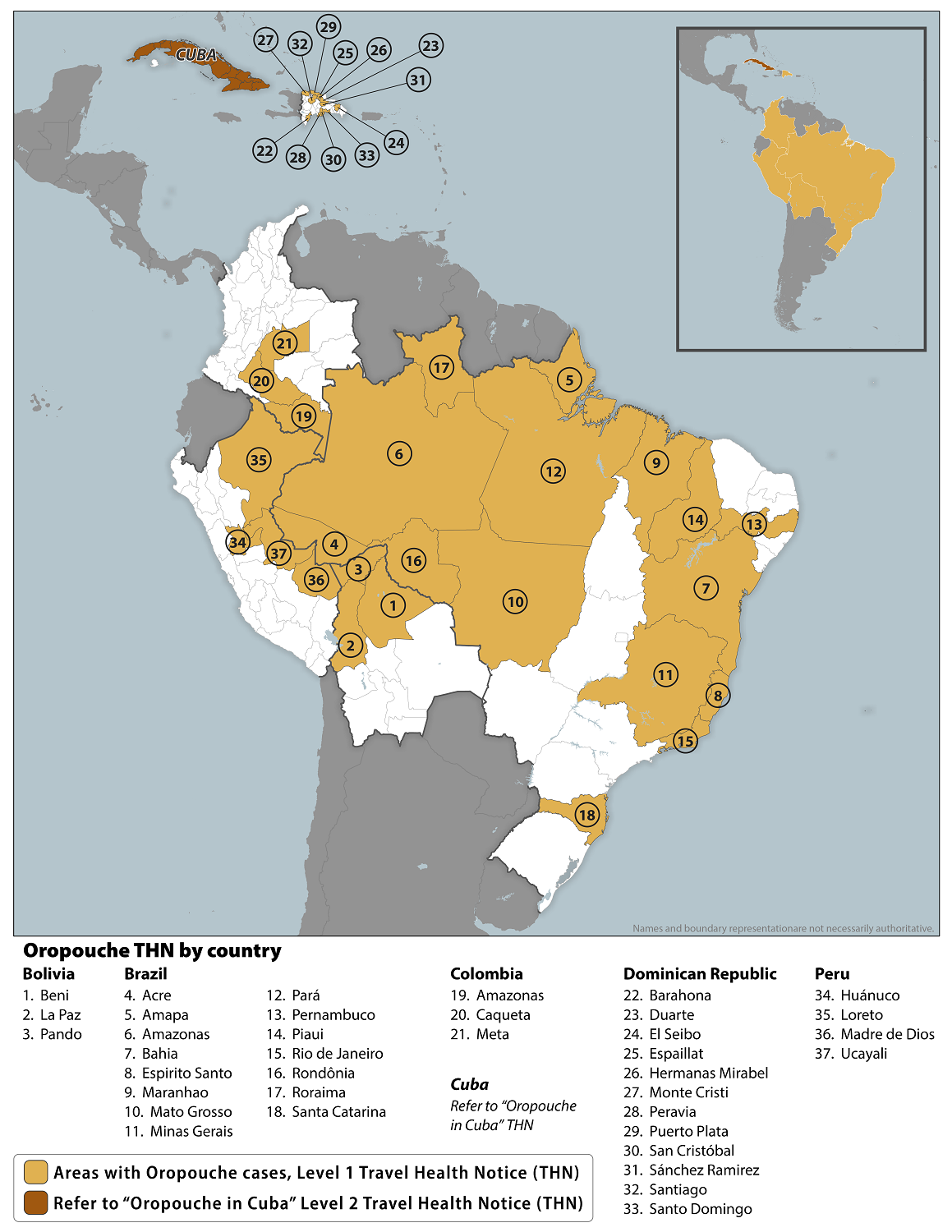
A recent study stressed the possible underestimation of Oropouche Fever (OF) cases and the potential global threat this arbovirus infection represents.
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed multiple cases of Oropouche in U.S. and European travelers returning from travel to Cuba, indicating an ongoing risk.
In the United States, Florida has reported 70 Oropouche cases in individuals with a travel history to an endemic area such as Cuba. The counties reporting cases are Broward (3), Duval, Hillsborough (6), Lee (2), Miami-Dade (28), Orange (2), Palm Beach (2), Pasco, Polk (2), and Sarasota.
As of September 29, 2024, the CDC has issued a Level 2 Travel Health Notice (THN) focused on Cuba's expanding Oropouche outbreak.
The CDC has issued a Level 1 THN for the Region of the Americas, identifying 37 areas at risk in Bolivia, Brazil, Colombia, the Dominican Republic, Guyana, and Peru.
Travelers to these affected areas should take steps to prevent insect bites, as the bite of infected midges and mosquitoes spreads Oropouche. This illness can occur in people of any age and is often mistaken for dengue fever.
Oropouche Virus (OROV; genus of Orthobunyavirus) is the causal agent of OF. Due to the lack of specific signs and symptoms and the limited availability of diagnostic tests, the actual epidemiology of OROV infections remains elusive.
While most infected people recover, there were also concerns about an increase in possible cases of the Oropouche virus being passed from a pregnant person to their fetus associated with fetal deaths and congenital abnormalities.
The CDC has confirmed no specific treatment or preventive vaccine for Oropouche exists.
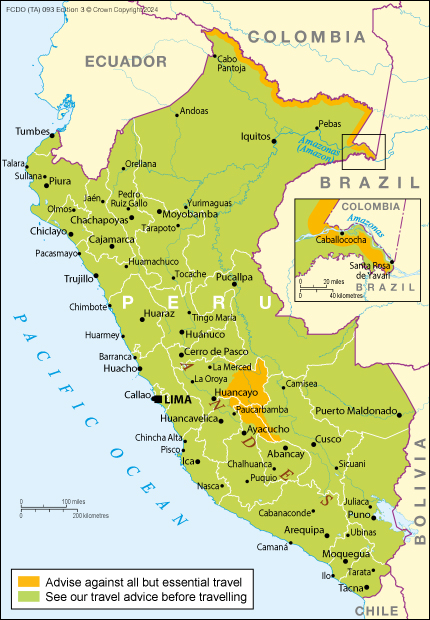
The U.K. Foreign, Commonwealth & Development Office (FCDO) recently issued a travel alert advising against all but essential travel to parts of Peru.
As of September 29, 2024, the FCDO posted new information regarding the Oropouche virus disease (OROV) and Yellow Fever outbreaks this year.
There is also a risk of infection when visiting Peru with Malaria and Zika virus through mosquito bites.
Over 2 million people visit Peru annually, visiting places such as Machu Picchu.
To alert international travelers to these health risks, the U.S. Centers for Disease Control and Prevention (CDC) reissued a Level 1 Practice Usual Precautions, Travel Health Advisory on September 25, 2024. The CDC advisory states that Oropouche fever is spread through the bites of infected midges (flies) and Culicoides paraenesis mosquitoes.
Seperately, a Level 2 Travel Health Notice has been issued for Oropouche outbreaks in Cuba.
While there are no specific medications or vaccines to prevent OROV, the risk of infection can be minimized by following bite avoidance measures when visiting areas with the infection. Treatment for Oropouche virus disease is supportive.
The FCDO and the U.S. CDC recommend that visitors to Yellow Fever endemic areas such as Peru and Brazil be vaccinated.
In the U.S., Sanofi Pasteur's YF-VAX® vaccine is offered at certified travel clinics and pharmacies.
The Minnesota Department of Health today announced it is investigating a human rabies death in a person who had exposure to a bat in western Minnesota in July 2024. This is Minnesota’s fourth case of human rabies since 2000.
This new rabies case is an essential reminder that bats can spread the virus and that the public should avoid contact with them. Bats with rabies are found in all U.S. states except Hawaii.
Recently, a resident of Brantford-Brant, Ontario, Canada, was also confirmed with bat-bite transmitted rabies.
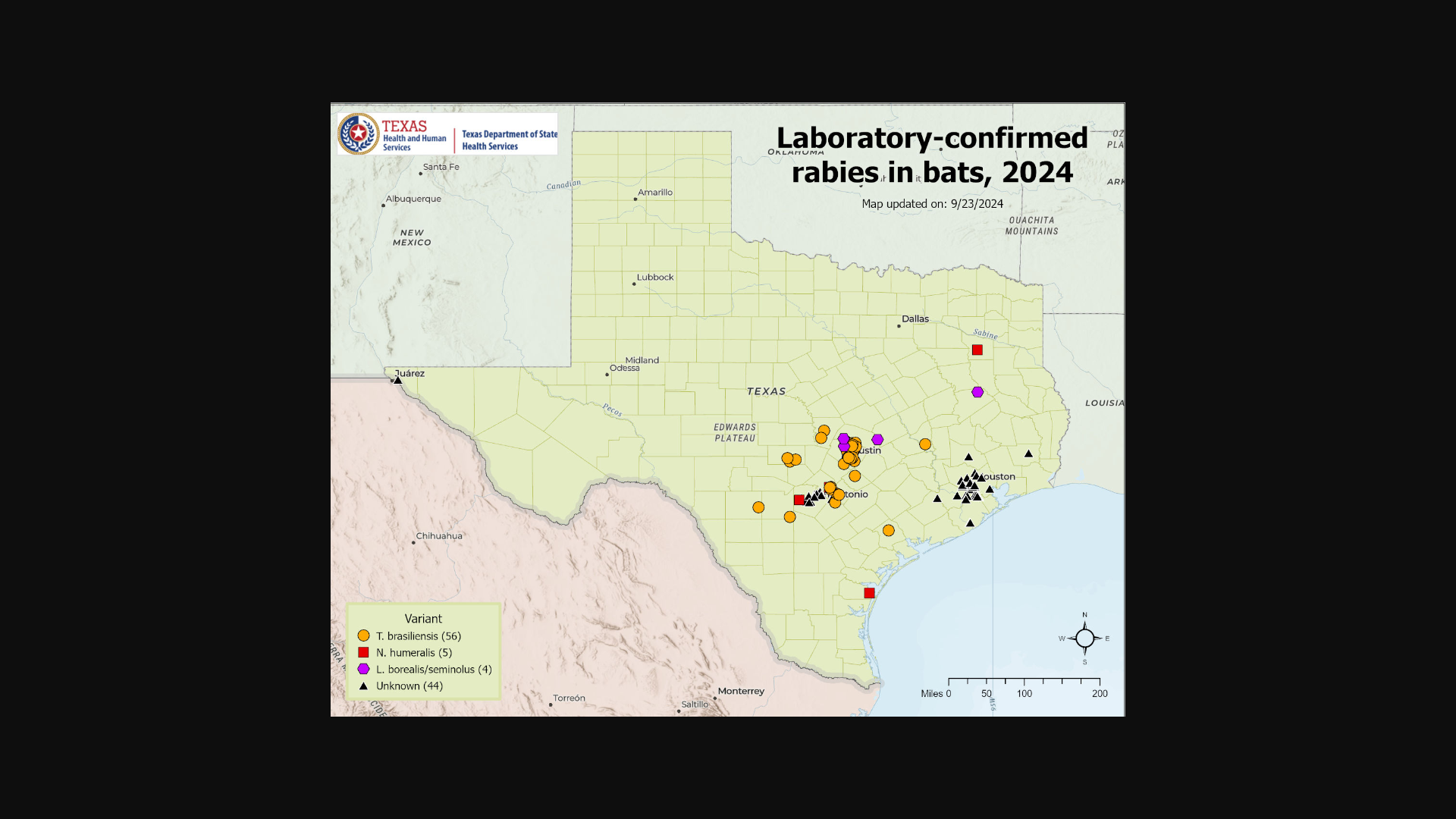
And in Texas, numerous bats have been confirmed with rabies in 2024.
Globally, rabies continues to claim about 59,000 lives annually, with Africa shouldering over 60% of these fatalities. Dogs, not bats, cause most rabies infections worldwide.
According to the U.S. CDC, rabies treatment (vaccines) has proven to be nearly 100% effective at preventing the disease after exposure, but it must be started before symptoms of rabies appear.
Left untreated, rabies is almost always fatal.
The CDC establishes recommendations for international travelers by evaluating the risk of rabies exposure and access to high-quality postexposure prophylaxis (PEP, including rabies immunoglobulin and vaccine) in each destination country.
Bavarian Nordic's RabAvert® vaccine is offered at many travel vaccine clinics and pharmacies in 2024.