Search API
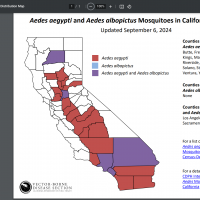
The Los Angeles County Department of Public Health confirmed today that locally acquired Dengue is emerging, which is extremely rare in a region where mosquitoes have not previously transmitted the virus.
LA is investigating a new case of locally acquired Dengue in an El Monte resident.
This case of local transmission is the fifth case of locally acquired Dengue reported in LA County's San Gabriel Valley (2 million population) this year.
Similar to the cases reported in Baldwin Park (3) and Panorama City (1), this person had no history of travel to areas where Dengue is endemic.
“This case further indicates that Dengue can spread in our community. Preventing mosquito bites and breeding is the best way to stop local dengue transmission,” said Muntu Davis, MD, MPH, Los Angeles County Health Officer, in a press release on October 3, 2024.
On the U.S. southeast coast, the state of Florida reported 548 travel-associated and 40 locally acquired dengue cases in 2024.
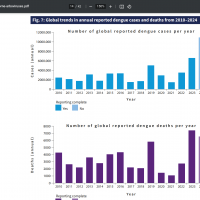
Throughout the Region of the Americas, over 11 million Dengue cases and 6,700 related fatalities have been confirmed in the Americas this year.
While Dengue is a vaccine-preventable disease, no vaccine is currently available in the U.S.
According to Reuters, Gilead Sciences today announced it would donate about 5,000 vials of its antiviral drug remdesivir (Veklury®) for emergency use in response to the ongoing Marburg virus disease (MVD) outbreak in the Republic of Rwanda.
This U.S. FDA-approved antiviral drug is being evaluated since no approved MVD vaccines are available.
However, several Marburg vaccine candidates, utilizing various technologies, are actively conducting clinical trials.
The drugmaker clarified that Remdesivir, originally developed to treat Ebola, is not approved for treating Marburg disease anywhere, and its safety and efficacy against the virus is unknown.
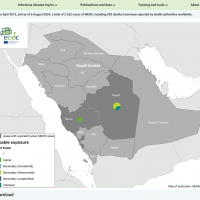
As of October 3, 2024, Rwanad's first MVD outbreak has produced eleven fatalities out of 36 confirmed cases.
The World Helath Organization recently assessed the risk of this MVD outbreak as very high at the national level, high at the regional level, and low at the global level. And the local government anticipates additional MVD cases to be reported this year.
The Republic of Rwanda Ministry of Health posted on X that the death toll from the recent Marburg virus disease (MVD) outbreak has reached eleven out of 36 confirmed cases.
As of October 2, 2024, about 26 additional people are in isolation from this rare viral hemorrhagic fever in Kigali, the capital city of Rwanda, which has a population exceeding 1.7 million.
As of September 30, 2024, the World Helath Organization (WHO) assessed the risk of this MVD outbreak as very high at the national level, high at the regional level, and low at the global level.
The WHO says the source of this MVD outbreak, geographical extent, likely onset date, and additional epidemiological information on cases are still pending further investigation. Rwanda declared its initial MVD outbreak on September 27, 2024.
According to the AP, Rwandan Health Minister Sabin Nsanzimana said that vaccine clinical trials would start “within days” but failed to clarify which type of vaccine will be used.
As of October 3, 2024, various Marburg vaccine candidates are conducting studies. For example:
Public Health Vaccines, LLC launched its Phase 1 clinical trial in March 2024, evaluating its single-dose vaccine candidate, PHV01 (rVSV∆G-MARV-GP [Angola]).
On April 15, 2024, the U.S. FDA granted orphan drug designation to the active ingredient in MarVax™, the subunit protein vaccine of recombinantly expressed MARV glycoprotein, for "the prevention and post-exposure prophylaxis against MARV infection.
The U.S. CDC's recent travel advisory remains in effect, which states If you travel to the Republic of Rwanda, you should:
Avoid contact with sick people with fever, muscle pain, and rash symptoms.
Avoid contact with blood and other body fluids.
Avoid visiting healthcare facilities in the outbreak area for nonurgent medical care or for non-medical reasons.
A new report, 'Advancing the Fight Against Neglected Tropical Diseases (NTDs) in Francophone Countries: Progress, Challenges, and the Path Forward for Sustainable Action,' was released today. It assesses the collective burden and progress of fighting NTDs in Francophone countries.
Published on October 3, 2024, the report highlights the unique opportunities within the Francophonie to leverage linguistic, cultural, and historical ties for collective action and cross-border collaboration to combat NTDs more effectively.
NTDs are a group of preventable and treatable illnesses caused by various pathogens, including viruses, bacteria, parasites, fungi, and toxins, and include Chagas, Chikungunya, Dengue, and Oropouche virus diseases.
Of the more than 1.6 billion people worldwide at risk of NTDs, a significant percentage reside in Francophone countries, particularly in sub-Saharan Africa and Southeast Asia.
French is an official language in 32 independent nations and 60 countries and territories where about 210 million people live. It is also a co-official or de facto language in many regions and organizations.
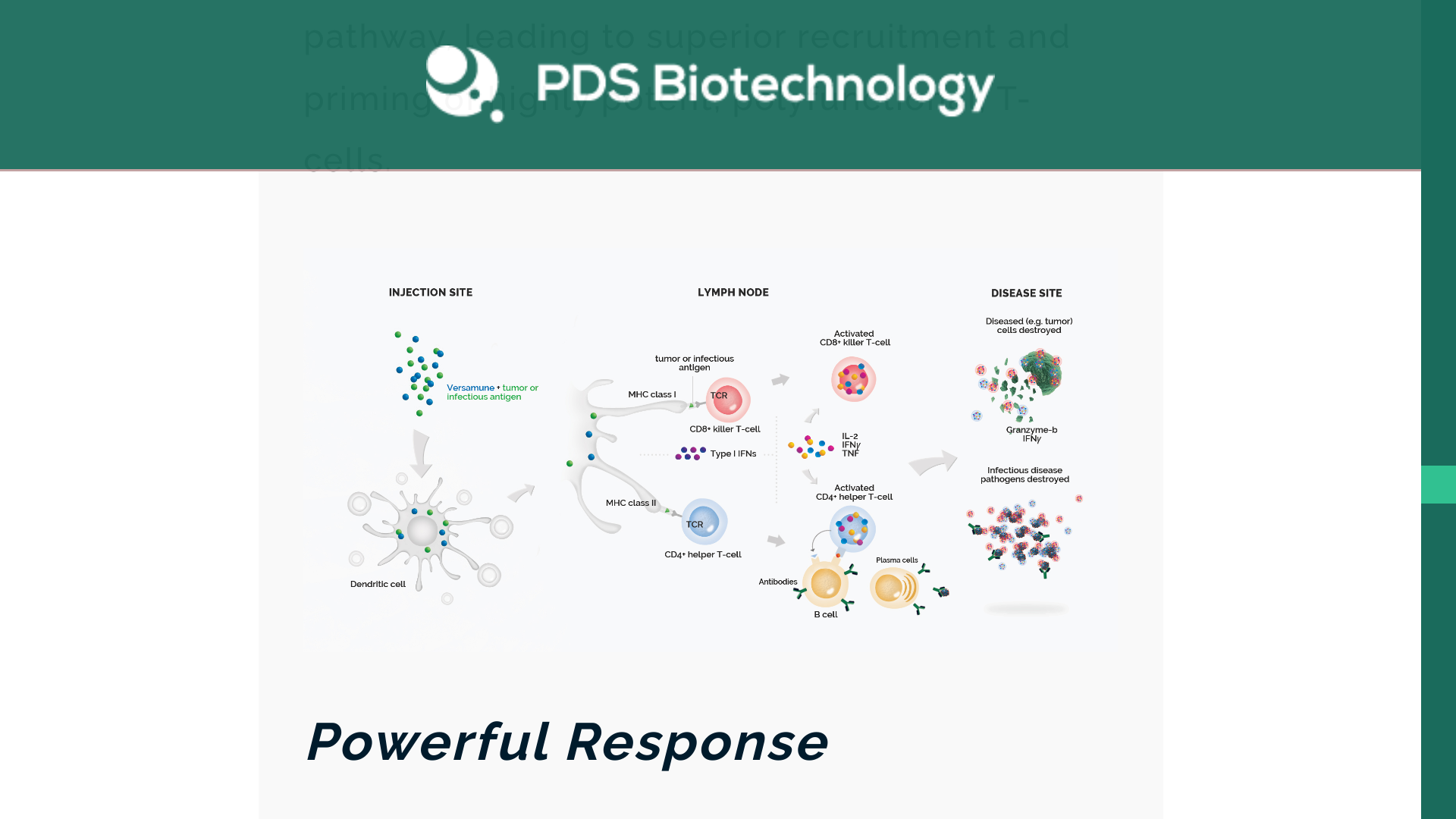
PDS Biotechnology Corporation today announced updated data from the Phase 2 clinical trial evaluating Versamune® HPV (PDS0101) with chemoradiation to treat locally advanced cervical cancer were presented at the American Society for Radiation Oncology Annual Meeting 2024.
The company stated 100% 36-month overall survival (OS) and progression-free survival (PFS) rates in patients fully treated with Versamune® HPV combined with chemoradiation (N=8), and 88% (15/17) of patients had a complete metabolic response.
“We are pleased that data from the Phase 2 IMMUNOCERV trial demonstrate compelling clinical activity and a promising safety profile,” said Frank Bedu-Addo, Ph.D., President and Chief Executive Officer of PDS Biotech, in a press release on October 2, 2024.
“Based on our continued research in various HPV-positive cancers, Versamune® HPV appears to work in combination with a variety of therapeutic agents to generate clinical responses and promote improved survival in patients with minimal toxicity. We look forward to the next steps in the development of Versamune® HPV for locally advanced cervical cancer.”
PDS Biotech’s oncology pipeline leverages the Versamune® platform and Versamune® plus PDS01ADC in combination with proprietary tumor-specific proteins or peptides (antigens) in developing targeted cancer immunotherapies.
The Republic of Rwanda's health ministry reported today that the sudden Marburg virus disease (MVD) outbreak had reached 29 cases and ten related fatalities.
On September 30, 2024, the World Health Organization confirmed MVD cases from seven of the 30 districts in Rwanda.
Over 70% of the confirmed cases are healthcare workers from two health facilities in Kigali, a city with about 1.5 million residents.
On September 27, 2024, the Rwanda Ministry of Health reported its first Marburg disease case.
Additionally, the U.S. CDC published a Travel Health Advisory to alert international travelers of this expanding health risk. Marburg virus is a Filovirus that, along with Ebola, can cause severe viral hemorrhagic fever infections. MVD was initially reported in 1967 during an outbreak in Marburg a der Lahn and Frankfurt am Main, West Germany.
As of October 1, 2024, no Marburg vaccine candidate has been approved to prevent infections in people.
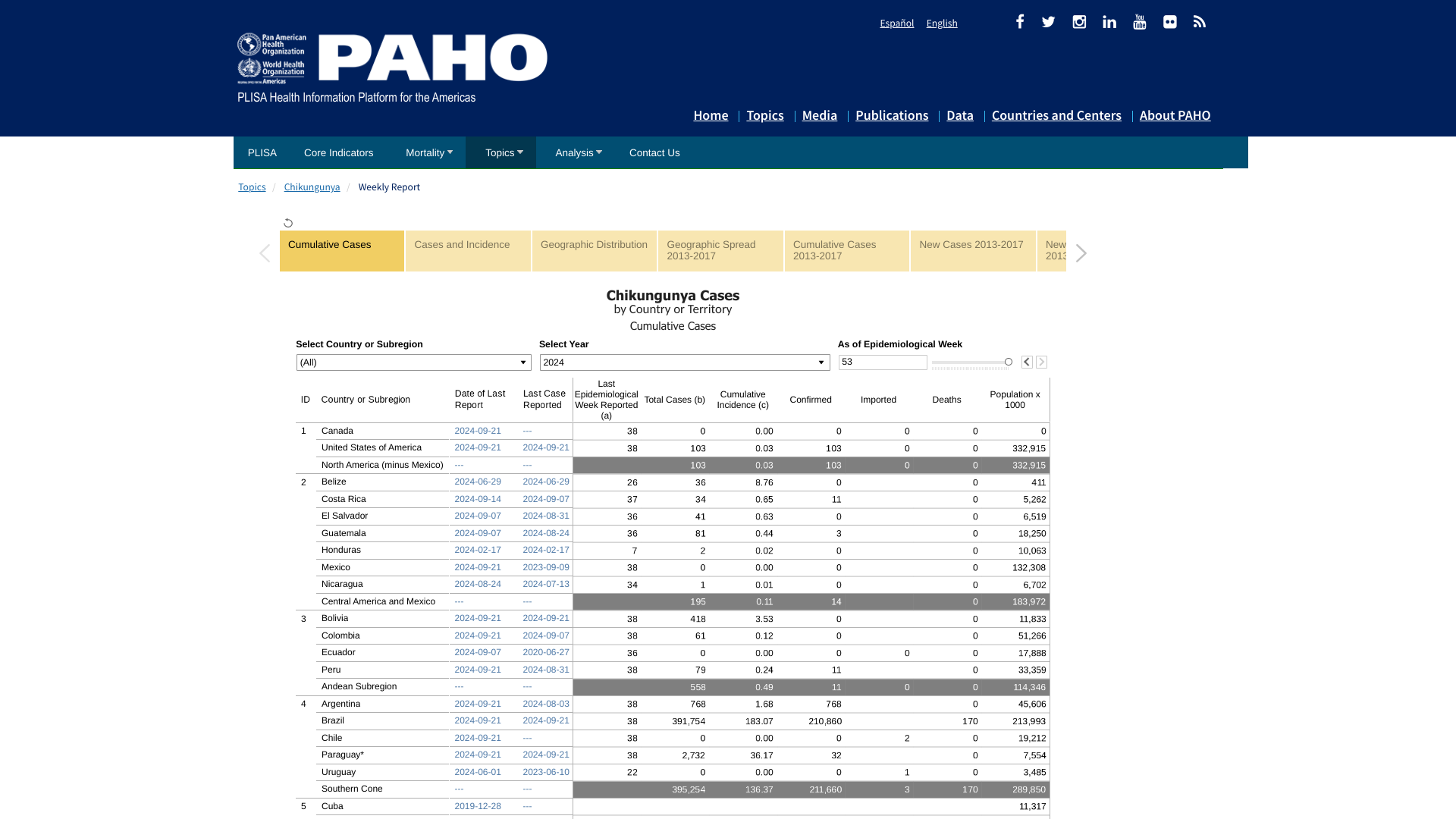
The Chikungunya Virus outbreak in the Region of the Americas has now exceeded 393,000 cases in 2024 and may soon surpass last year's record of 411,548.
Throughout 2024, the Pan American Health Organization has reported 170 Chikungunya-related fatalities in the Americas.
Before 2006, Chikungunya, a mosquito-transmitted virus, was rarely identified in U.S. travelers. However, that trend has changed,
The Centers for Disease Control and Prevention (CDC) data dashboard reported 113 chikungunya cases in 2024, an increase of 34 patients since September 2024. Over 20 states have reported Chikungunya cases this year, led by Massachusetts, New Hampshire, California, Colorado, Illinois, and New York.
The CDC did confirm some good news today; these confirmed cases are travel-related.
Locally acquired Chikungunya cases have not been reported from U.S. states or territories since 2019.
Furthermore, the CDC says all travelers to countries with a risk of Chikungunya should avoid mosquito bites and discuss the need for vaccination with their healthcare provider.
As of October 2024, the Valneva SE's IXCHIQ® single-dose Chikungunya vaccine is readily available at travel clinics and pharmacies in the U.S.