Search API
Millions of people will be infected with the Dengue virus in the last few months of 2024 without being able to be treated with an approved antiviral.
Johnson & Johnson (J&J) announced on October 4, 2024, that it discontinued the Phase 2 field study evaluating the efficacy of investigational antiviral candidate mosnodenvir (JNJ-1802) for the prevention of dengue virus in adults.
Recent results from the Phase 2a human challenge study found that the compound induced antiviral activity against one of dengue's four viruses (DENV-3) in humans, compared to placebo.
J&J's decision to discontinue this study is part of a strategic reprioritization of the Company’s Communicable Diseases research and development portfolio. However, J&J will continue to support the fight against dengue by sharing study results with the medical community in the future.
Almost 4 billion people will live in areas at risk of dengue in 2024, including 26 countries recently highlighted by the U.S. Centers for Disease Control and Prevention (CDC).
According to the U.S. CDC, dengue symptoms can become severe within a few hours. See a healthcare provider if you develop a fever or have symptoms of dengue. Dengue can be found in the blood during the first week of illness.
Furthermore, time is of the essence as severe dengue is a medical emergency.
Dengue is a vaccine-preventable disease. Various approved vaccines have limited availability, and candidate vaccines are conducting late-stage research in October 2024.
According to the Pan American Health Organization (PAHO) chikungunya data dashboard, the Federative Republic of Brazil has reported 16 additional fatalities related to chikungunya virus (CHIKV) disease outbreaks in one month.
As of October 6, 2024, Brazil has confirmed 177 fatalities and 394,739 CHIKV cases this year.
Data from Brazil's Ministry of Health indicates that Minas Gerais has reported the most chikungunya cases, over 160,000, followed by Mato Grosso and Bahia.
Minas Gerais is Brazil's fourth-largest state by area, with a population exceeding 20 million.
In 2023, Brazil welcomed about 6 million foreign tourists, many visiting Minas Gerais.
The U.S. CDC confirmed in September 2024 that there has been evidence of chikungunya virus transmission in Brazil within the last five years, and vaccination may be considered for certain visitors.
As of October 6, 2024, Valneva SE's IXCHIQ® chikungunya vaccine has been approved by the U.S. Food and Drug Administration and recommended for travelers to endemic areas by the CDC.
IXCHIQ is commercially available at travel clinics and pharmacies in the U.S.
The European CDC recently reported there have been no significant changes in the global circulation of monkeypox virus (MPXV) clade I and clade II during the past week.
In 2024, over 34,000 confirmed and suspected mpox cases due to MPXV clade I and clade II, including over 850 deaths, have been reported from Africa.
To slow the spreading of clade I, the Democratic Republic of the Congo (DRC) announced today vaccination efforts to halt the spread of mpox disease.
The DRC received 265,000 doses of the MVA-BN (Bavarian Nordic A/S, JYNNEOS®) vaccine donated by the European CCommission'sHealth Emergency Preparedness and Response Authority, Gavi, the Vaccine Alliance, and the United States Government.
Vaccinations will be launched in the eastern North Kivu province on October 5, 2024, and will prioritize health workers and frontline responders, contacts of confirmed cases, contacts of those contacts, and other at-risk groups.
Subsequently, the vaccination will be rolled out in eleven of the most affected health zones in Equateur, North Kivu, Sankuru, South Kivu, Sud-Ubangi, and Tshopo provinces.
With a population of about 100 million and assuming two doses per person, 130,000, or 1% of the people in the DRC, can be better protected from mpox.
"The rollout of the vaccine marks an important step in limiting the spread of the virus and ensuring the safety of families and communities,” said Dr. Matshidiso Moeti, WHO Regional Director for Africa, in a WHO press release.
Person-to-person transmission of the MPXV has occurred during this outbreak, including through sexual contact, day-to-day household contact, and within the healthcare setting.
Mpox vaccination is now recommended for most people visiting outbreak areas, says the ECDC. There are now several Mpox vaccines available worldwide.
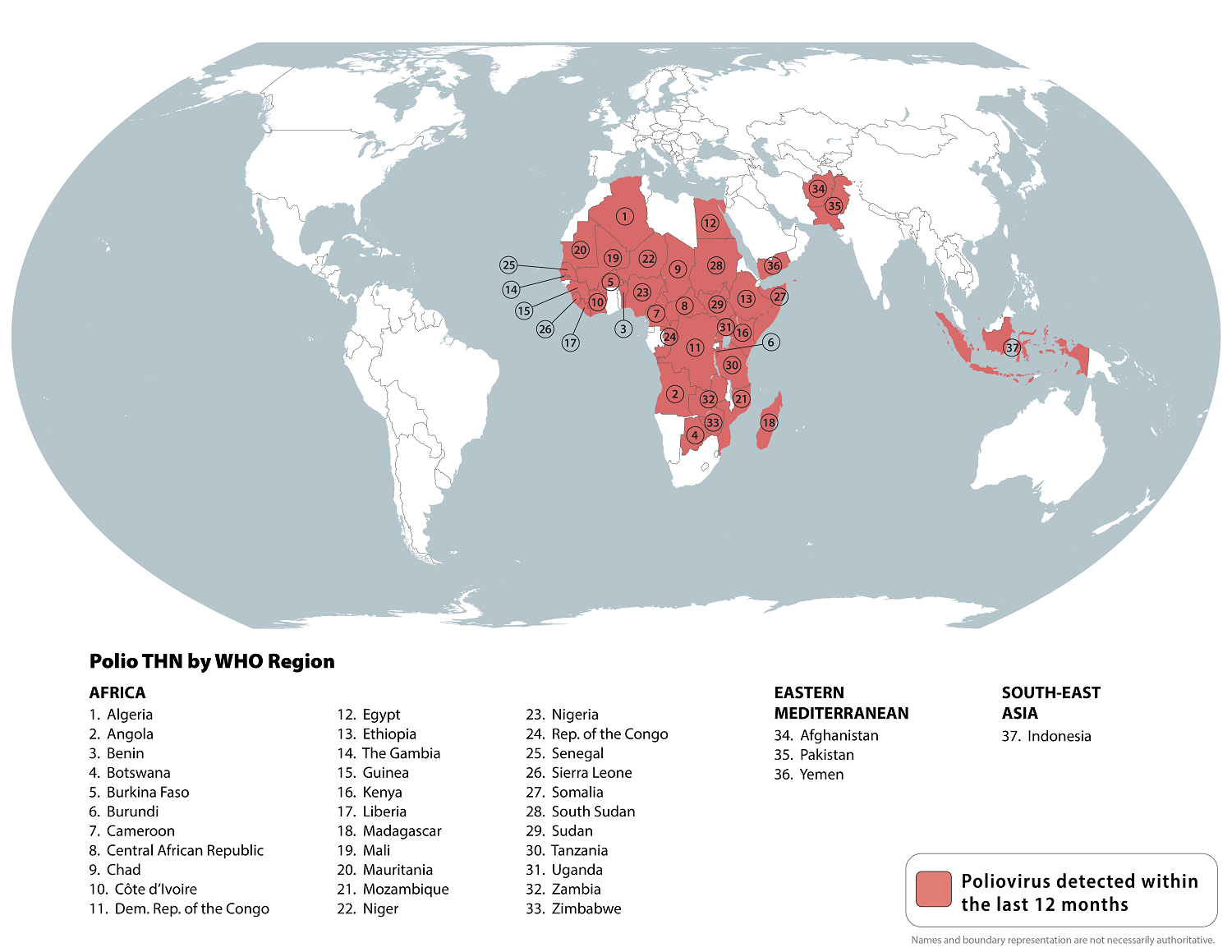
While the World Health Organization continues alerting international travelers to ongoing polio risks, two countries are the key focus. As of October 5, 2024, polio remains endemic in Pakistan and Afghanistan.
Unfortunately, two additional children were paralyzed by wild poliovirus type 1 in Pakistan, underscoring the expanding public health threat.
According to the Regional Reference Laboratory at the National Institute of Health, a female child from the Zhob district of Balochistan and a male child from the Tank district of Khyber Pakhtunkhwa have been affected,
This update indicates the total number of polio cases in 2024 is now 28.
The Coordinator of the National Emergency Operations Center for Polio Eradication, Mr. Muhammad Anwarul Haq, stated that consistent polio campaign implementation in Balochistan and southern KP has been challenging for the Programme since last year due to factors such as lack of access to vulnerable children, security issues, vaccine hesitancy, and community boycotts.
“Access challenges leave a cohort of unvaccinated children who have low immunity to fight off a polio infection. The Polio Programme is working closely with both provincial governments to increase vaccination coverage rates, build community trust, and provide integrated health service delivery for better health outcomes,” he said in a press release on October 2, 2024.
Polio is a highly infectious disease caused by the poliovirus, mainly affecting children under five. It invades the nervous system and can cause paralysis or even death. While there is no cure for polio, vaccination is the most effective way to protect children from this crippling disease, says the Global Polio Eradication Initiative (GPEI).
The U.S. CDC recently published a Travel Health Advisory that identified 37 destinations that have circulating poliovirus.
Before any international travel, make sure you are up to date on your polio vaccines, says the CDC. Adults who previously completed the routine polio vaccine series may receive a single, lifetime booster dose of the IPV polio vaccine.
Polio vaccination services are offered at travel vaccine clinics and pharmacies in the U.S.
Earlier in 2024, countries and partners committed nearly $600 million in new funding towards elimination of cervical cancer. About 95% of the 660,000 cervical cancer cases occurring globally each year are caused by the human papillomavirus (HPV).
To expand access to HPV vaccines, the World Health Organization (WHO) announced that a fourth WHO-prequalified vaccine, Cecolin®, has been confirmed for use in a single-dose schedule.
A growing number of HPV vaccine products initially prequalified for use in a 2-dose schedule can now be used in a single-dose schedule.
“Unlike most other cancers, we can eliminate cervical cancer, along with its painful inequities,” said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, in a press release on October 4, 2024.
“By adding another option for a one-dose HPV vaccination schedule, we have taken another step closer to consigning cervical cancer to history.”
The WHO also confirmed an additional HPV vaccine, Walrinvax®, is now the fifth product available on the global market. Walrinvax® is prequalified with a two-dose schedule. Further data will be needed to assess if this vaccine can be recommended for single-dose schedules in the future, says the WHO.

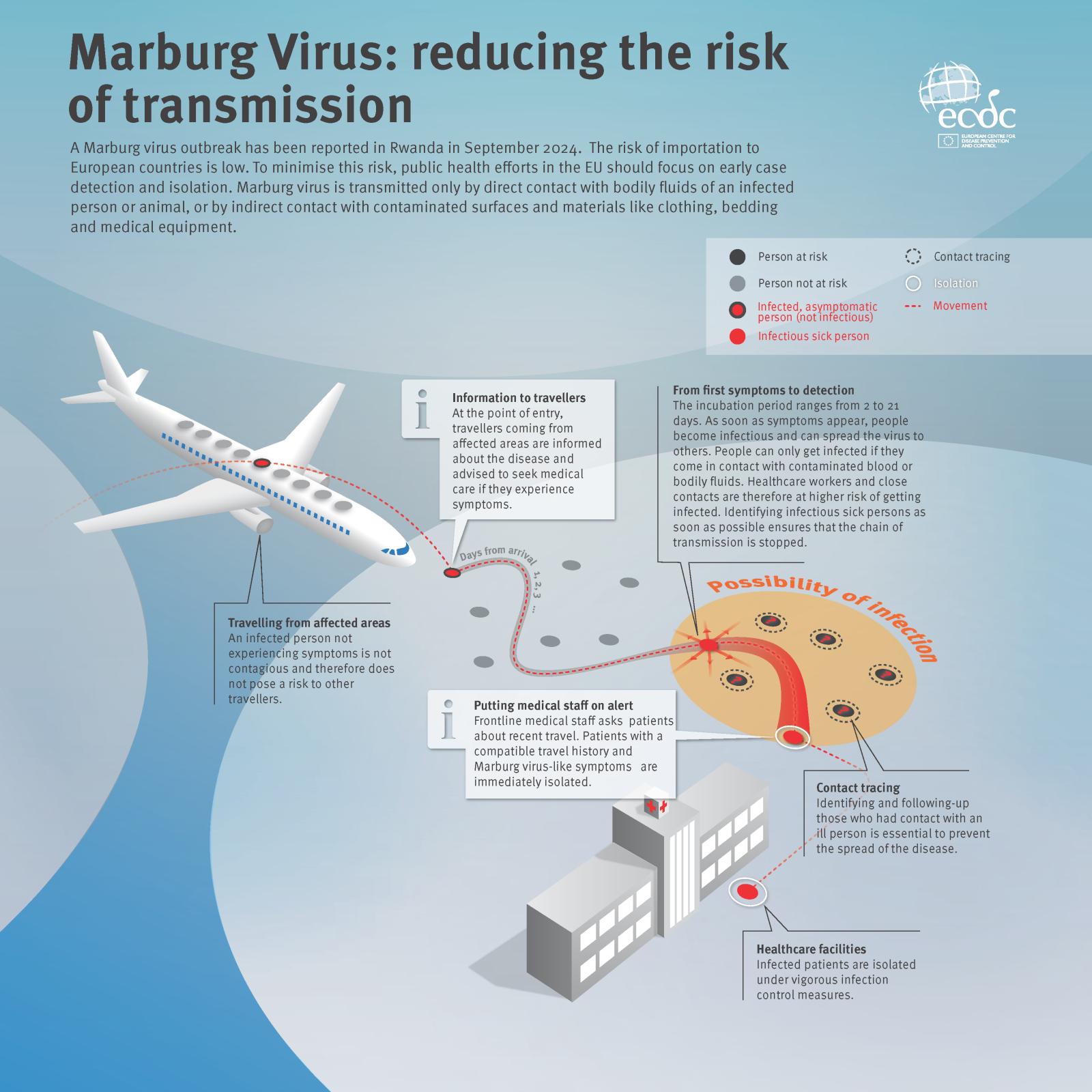
Based on the Ministry of Health guidelines, the Republic of Rwanda's Development Board provided the following update on Marburg Virus Disease (MVD).
This viral hemorrhagic fever has claimed 12 lives from 41 cases over the past month.
As of October 4, 2024, no travel restrictions are currently in place, and all key tourist destinations, including Volcanoes National Park, Akagera National Park, Gishwati Mukura National Park, and Nyungwe National Park, are fully operational.
However, at points of entry, visitors should expect routine temperature checks and the provision of hand sanitizing stations at airports and other border crossings.
All people exiting Rwanda will undergo an MVD symptom screening questionnaire to ensure their well-being and the safety of others.
And tourists to this African country are advised to remain vigilant and practice good personal hygiene, such as avoiding close contact with individuals displaying symptoms such as high fever or severe headaches.
The U.S. CDC published an updated travel advisory on October 3, 2024, highlighting actions to prevent contracting MVD while visiting Rwanda.
As of today, no approved MVD vaccines are available.
According to a research letter published in the Journal of the American Medical Association (JAMA), Bavarian Nordic’s mpox vaccine JYNNEOS® (MVA-BN®) protection appears to weaken within six to 12 months after injection.
Two doses provided 66% effectiveness, and one dose provided 36% effectiveness at peak immunity during the 2022 clade 2 mpox outbreak.
This finding raises questions about whether the two-dose vaccine may been 'booster' shots to confer lasting protection against the mpox virus.
However, the U.S. CDC wrote on September 12, 2024, 'Right now, getting more than two mpox vaccine doses (a "booster") isn't recommended.'
“Our data suggest that protective immunity may be waning in individuals who were vaccinated with this vaccine in 2022,” corresponding author Dan Barouch said in a related statement on October 3, 2024.
On September 26, 2024, Bavarian Nordic announced an agreement with UNICEF to supply 1 million doses of the MVA-BN® mpox / smallpox vaccine for African countries confronting the clade 1 mpox outbreak.
In the United States, JYNNEOS is now commercially available at clinics and pharmacies.