Search API
A research letter recently published by The NEJM assessed the serostatus of asylum seekers in New York City.
On November 20, 2024, the researchers disclosed that about 27% of the participants in a study were seronegative for measles. This data indicates these people did not have protective antibodies against this highly contagious disease.
As of late November 2024, the U.S. CDC reported eight imported and six locally-acquired measles cases have been reported in New York City this year.
Nationwide, the CDC reported 277 measles cases in 32 jurisdictions as of November 7, 2024. Measles outbreaks have been declared in sixteen areas this year.
If one person has measles, up to 90% of people around them will also get it if they are unvaccinated or otherwise not immune.
'Anyone who has received two doses of a measles vaccine is considered immune for life and unlikely to get measles,' wrote NYC Health.
Since measles is a vaccine-preventable disease, most pharmacies in New York offer measles vaccination services. Call 311 for information on where you or your child can get vaccinated.
While the CDC has not issued a Travel Health Advisory focused on New York City, the U.K. says travelers to the U.S. should be up to date with routine vaccinations, including the measles, mumps, and rubella vaccine.

Dyadic International, Inc. announced today that it has been awarded a $3 million grant from the Gates Foundation for the cell line development of monoclonal antibodies (mAbs) targeting respiratory syncytial virus (RSV) and malaria utilizing the company's proprietary C1 protein production platform to provide globally accessible treatment options for underserved populations.
Mark Emalfarb, Founder and CEO of Dyadic, stated in a press release on November 21, 2024, "We believe C1's increased efficiency and cost-effectiveness can expand access to therapeutics and vaccines for populations impacted by health disparities."
The versatile C1-cell protein production platform is based on an industrially proven microorganism (C1) designed to accelerate development, reduce production costs, and improve the scalability and performance of biologic vaccines and therapeutics for both human and animal health markets.
Currently, the C1 platform is being utilized in collaborations with leading pharmaceutical, biotech, academic, and government organizations to develop innovative vaccines and treatments.
If these research efforts succeed, Dyadic plans to commercialize these and other antibodies through licensure, expanding access to affordable treatment options for patients worldwide and reducing the global burden of infectious diseases.
As of late November, U.S. FDA-approved RSV mAbs (Beyfortus™) are in full supply and are offered to newborn infants in the U.S.
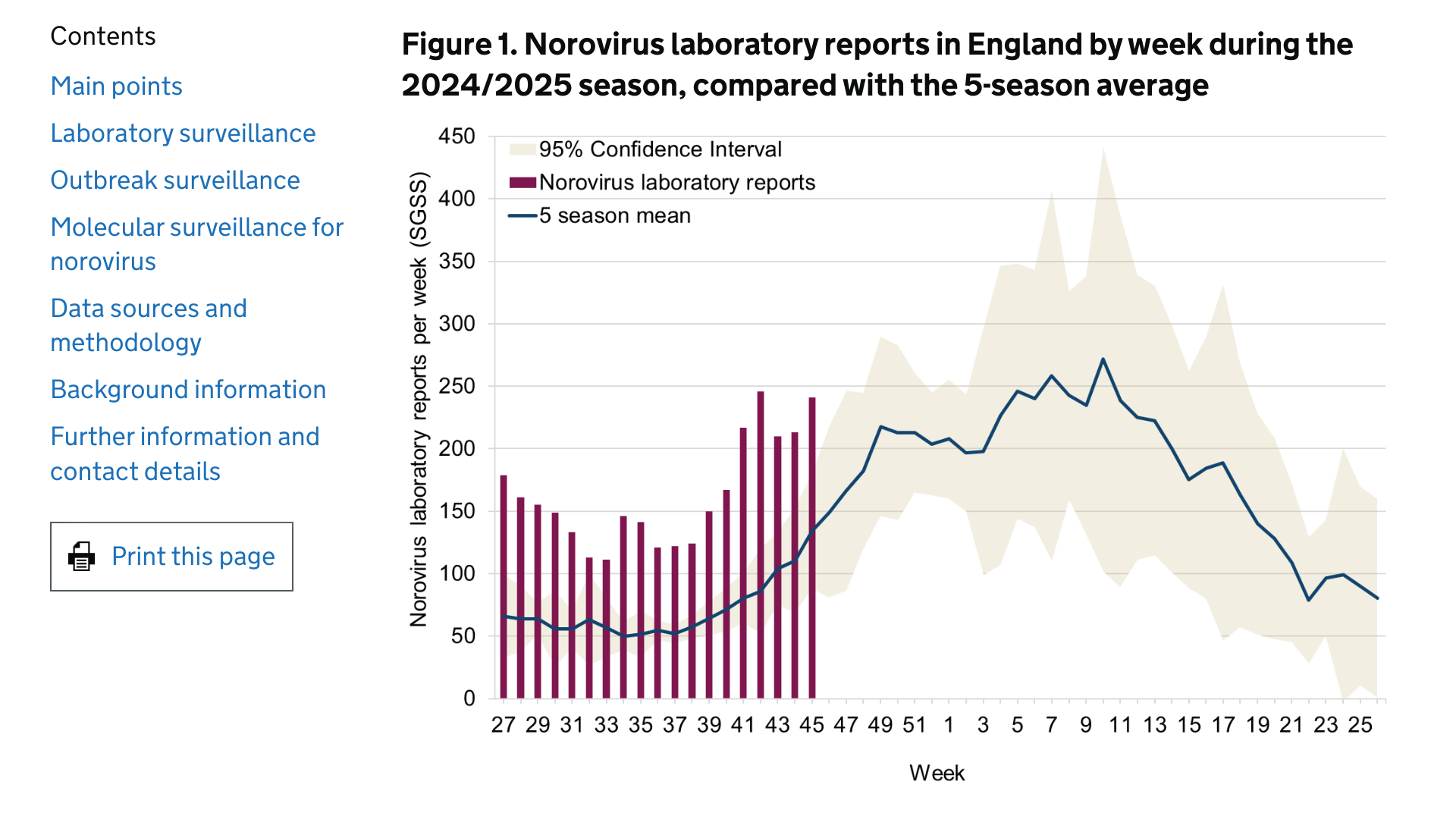
The U.K. Health Security Agency says the timing of the typical norovirus seasonal increase and peak of activity varies from season to season.
This season, the increase in reporting has begun earlier than in the last five seasons.
As of November 21, 2024, data derived from the Second-Generation Surveillance System up to week 45 of the 2024/2025 season showed that the cumulative number of positive norovirus laboratory reports in England (3,099 laboratory reports) was more than double the 5-season average for the same period (1,342 laboratory reports).
The most commonly detected norovirus genotype worldwide is genogroup II—genotype 4 (GII.4). Historically, there have been five global GII.4 strain replacement events.
While norovirus vaccines are in demand, developing a broadly effective vaccine remains difficult, owing to noroviruses' genetic and antigenic diversity. However, one vaccine candidate continues pursuing U.S. FDA approval.
In the U.S., HilleVax, Inc.'s HIL-214 virus-like particle bivalent vaccine candidate is designed to prevent moderate-to-severe acute gastroenteritis caused by norovirus. HIL-214 vaccine includes antigens targeting genotypes GI.1 and GII.4.
As of November 2024, HIL-214 has been studied in nine clinical trials but has not been approved for commercial use.
In November 2024, a sample of municipal sewage collected in Warsaw, Poland, revealed the presence of wild poliovirus type 2.
Initial analysis suggests it is linked to the cVDPV2 emergence originating in Zamfara, northern Nigeria. This year, it has been detected internationally, including recently in Barcelona, Spain.
Poland's health ministry wrote on November 18, 2024, 'This disclosure does not indicate disease among humans, but according to WHO guidelines, suggests the need to take preventive measures.'
In connection with this incident, the State Sanitary Inspectorate took several actions, including updating poliomyelitis vaccine stock levels. In 2023, the vaccination rate of children aged 3 in Poland was 86%.
The last poliovirus detections in Poland were recorded in 1982 and 1984.
'However, due to cases of this vaccine-preventable disease in Asia and Africa, continuous surveillance is being carried out in Warsaw for flaccid paralysis cases, and municipal sewage is being tested for poliomyelitis viruses,' added the ministry.
According to the Global Polio Eradication Initiative, similar poliovirus detections have recently occurred in various countries. For example, Pakistan reported additional wild poliovirus type 1 cases, increasing the total cases to 50 in 2024.
When the U.S. CDC updated its Level 2—Practice Enhanced Precautions, Travel Health Advisory, in August 2024, Poland was not among the 37 countries that had reported poliovirus issues.
The CDC says that before traveling to any of the listed destinations, adults who have previously completed the entire routine polio vaccine series may receive a single lifetime booster dose of the polio vaccine. Regardless of age, anyone who has not been vaccinated can get the disease.
Polio vaccines are offered at health clinics and pharmacies in the U.S.
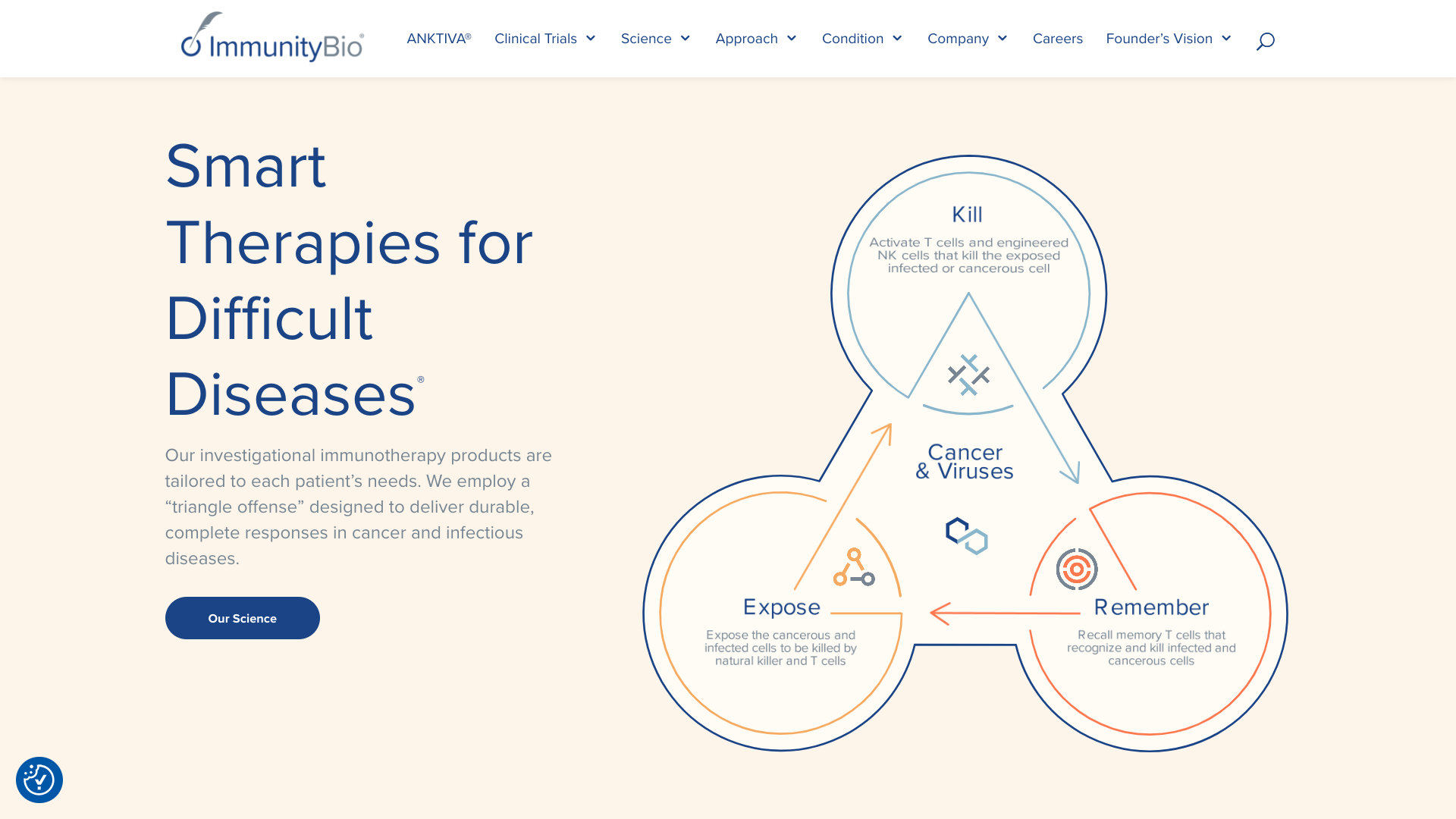
ImmunityBio, Inc. recently announced compelling new data from its ongoing QUILT 3.032 study. As of November 2024, 100 patients with Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer with carcinoma in situ (NMIBC CIS) have been treated with ANKTIVA® (nogapendekin alfa inbakicept-pmln) in combination with BCG, achieving a 71% complete response rate.
This significant milestone underscores the potential of ANKTIVA to provide durable responses in patients with limited treatment options.
In these responders, the range of durable response extended to 54 months.
Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio, commented in a press release on November 19, 2024, "These results highlight the potential of ANKTIVA to transform the treatment landscape for patients with BCG-unresponsive NMIBC CIS, offering hope for improved outcomes and cystectomy avoidance, especially with the prolonged duration of response now ranging as much as 54 months in this 100-patient analysis."
"Duration of complete response is the key efficacy element in driving cystectomy avoidance in this BCG-unresponsive population."
"I am pleased that this updated ANKTIVA data confirms that one of the highest durable responses is achieved compared to other approved products in this indication."
According to the company, this data update will be submitted to the European Medicines Agency in a Marketing Authorization Application for ANKTIVA in the European Union, which is anticipated during Q4 2024.
In 2024, the U.S. Food and Drug Administration (FDA) approved ANKTIVA plus BCG for treating patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors. The FDA has also approved Merck's TICE® BCG vaccine for this therapy.
Uvax Bio, LLC today announced the interim analysis results from its first Phase 1 clinical trial evaluating the Company’s HIV-1 vaccine candidates, UVAX-1107 and UVAX-1197.
In the first stage of this trial, the subjects received either UVAX-1107 adjuvanted with CpG 1018® and aluminum hydroxide or placebo.
UVAX-1107 was immunogenic and generated robust IgG responses to the vaccine antigen derived from an HIV-1 strain known as BG505. 100% of subjects in the vaccine group demonstrated antibody responses after two priming vaccinations with UVAX-1107.
Antibody response titers increased >200-fold 14 days after the 2nd dose compared to the same period following the first dose.
“We are pleased that our first Phase 1 trial is progressing smoothly, and we have preliminary confirmation that UVAX-1107 was well tolerated at all doses by the study participants, and no vaccine-related serious adverse events were reported,” said Pedro Garbes, M.D., Vice President and Global Clinical Lead of Uvax Bio, in a press release on November 19, 2024.
“As per the time of this 1st interim analysis, no participant was withdrawn from the study due to local/systemic reactogenicity; local and systemic adverse events were mild to moderate, transient, and resolved on average within two days. These preliminary safety results are aligned with expectations for an adjuvanted protein-based vaccine.”
UVAX-1107 utilizes Uvax Bio’s 1c-SApNP® vaccine development platform to generate virus-like particles that closely resemble the target virus in size, shape, and multivalent antigen display; in this case, 20 copies of the native-like, prefusion-stabilized trimeric HIV-1 antigen.

The World Health Organization (WHO) today announced it has granted Emergency Use Listing (EUL) for the LC16m8 smallpox / mpox vaccine, making it the second mpox vaccine to be supported by WHO.
LC16m8 is a vaccine developed and manufactured by KM Biologics in Japan.
It is recommended by the WHO for use in individuals over one year of age as a single-dose vaccine via a multiple puncture technique using a bifurcated needle.
“WHO emergency use listing of the LC16m8 vaccine against mpox marks a significant step in our response to the current emergency, providing a new option to protect all populations, including children,” commented Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, in a press release on November 19, 2024.
“Vaccines are one of the important tools to help contain the outbreak as part of a comprehensive response strategy that also includes improved testing and diagnosis, treatment and care, infection prevention control, and engagement and education within affected communities.”
This decision is expected to facilitate increased and timely vaccine access in communities where mpox outbreaks are surging.
Today’s move is particularly relevant as the Government of Japan has announced that it will donate 3.05 million doses of the LC16m8 vaccine and specialized inoculation needles to the Democratic Republic of the Congo. This is the most extensive vaccine donation package announced to date in response to the current mpox emergency.
Previously, the WHO has LIsted Bavarian Nordic A/S JYNNEOS® (MVA-BN®) smallpox / mpox vaccine.