Search API
Most older adults and immunocompromised individuals are familiar with herpes zoster (HZ), which causes painful rashes upon activating the varicella-zoster virus (VZV).
Although the U.S. FDA has approved a vaccine (Shingrix®) for preventing shingles, its administration is commonly associated with high reactogenicity.
On March 14, 2025, results from a new study published by the journal Nature focused on ten different vaccine candidate designs using two different codon optimizations targeting the VZV glycoprotein E (gE).
For this evaluation, researchers developed several VZV modRNA vaccine candidates targeting the glycoprotein gE, one of the most abundant proteins on the surface of the virion.
A subset of mRNA constructs was formulated into lipid nanoparticles and assessed for their ability to induce specific cellular and humoral immune responses in mice following vaccination.
Notably, the selected mRNA vaccine candidates induced high antibody levels and robust CD4+ and CD8+ immune responses.
Moreover, this study showed that alternate lyophilized vaccines provide comparable immunogenicity to liquid frozen formulations and are stable under long-term storage conditions.
Some of these investigational VZV modRNA candidates, including a lyophilized presentation, are currently being tested in a Phase I/II clinical study sponsored by Pfizer Inc.
This study's primary completion estimate is in late 2025.
While somewhat similar, no herpes simplex virus (HSV) vaccines are approved for use in 2025. However, this is an HSV mRNA vaccine candidate conducting research as of March 2025.

Each flu season, government agencies meet to review potential changes to influenza vaccines. These reviews are essential since influenza viruses often change during the migration between the Southern and Northern Hemispheres.
This means influenza vaccines must be updated annually to include the viruses that will most likely circulate in the upcoming flu season.
Today, the U.S Food and Drug Administration (FDA) announced its recommendations to vaccine manufacturers for the virus strains to be used in influenza (flu) vaccines for the 2025-2026 flu season in the United States.
On March 13, 2025, the FDA stated that after a comprehensive review of U.S. and global surveillance data, the recommendations are similar to those for the previous year’s strain selection.
Furthermore, the FDA does not anticipate any impact on the timing or availability of vaccines in the U.S. This year, about 100 million flu shots were distributed in the U.S.
To inform the selection of the flu virus strains, the FDA convened a meeting of scientific and public health experts from the FDA, Centers for Disease Control and Prevention, and Department of Defense. During the meeting, these federal partners collaboratively evaluated and analyzed U.S. and global surveillance data related to the epidemiology and antigenic characteristics of flu viruses currently circulating.
For the ongoing 2024-2025 flu season, most vaccines are trivalent (three-component), and many will be thimerosal-free and egg-based. These flu shots are offered at clinics and community pharmacies in the U.S.

Since the U.K. Health Security Agency (UKHSA) declared a national measles incident in parts of England in 2024, cases have been reported in various cities besides London.
So far in 2025, 151 measles cases have been confirmed in Yorkshire and Humber, in the South West, Bristol, Leeds, Hertfordshire, and in London.
As of March 13, 2025, no acute measles-related deaths have been reported in 2025.
Last year, the UKHSA reported the most measles cases (2,911) in England in over a decade.
To England's east, 127,350 people were diagnosed with measles in Europe in 2024, led by Romania (27,568). The WHO says this is the highest number of measles cases in over 25 years.
“Measles is back, and it’s a wake-up call.... without high vaccination rates, there is no health security,” warned Dr Hans Henri P. Kluge, WHO Regional Director for Europe, in a media statement.
“The measles virus never rests – and neither can we.”
While the U.S. Centers for Disease Control and Prevention (CDC) recent Travel Health Notice identifies 57 countries reporting measles cases, it does not mention England.
However, the CDC does suggest that anyone visiting a measles outbreak area be fully protected from this contagious virus with the MMR vaccine, which is generally available at clinics and pharmacies in the U.S.

As Chikungunya outbreaks continue to expand globally in 2025, France's La Réunion Islanders are urged to be vigilant in eliminating breeding sites.
According to France's Ministry of Health on March 12, 2025, 1,766 new Chikungunya cases were recorded last week. This represents an 18% increase compared to the previous week.
During 2025, 5,041 cases have been reported.
La Reunion's southern municipalities have been the most affected, accounting for 72% of cases since August 2024.
The last Chikungunya epidemic in La Réunion was reported in 2005–2006.
France's island off the east coast of Africa is not alone in reporting cases. In 2024, locally transmitted Chikungunya infections were confirmed in Paris and France's Mediterranean coastal communities.
These areas in France have also reported Dengue and Zika infections, which are also mosquito-transmitted diseases.
Also located in the South Hemisphere, Brazil has had a Chikungunya outbreak again this year, with over 40,000 cases, many centered in its mountainous states.
To alert international travelers to these outbreaks, the U.S. CDC has issued a Travel Health Advisory and suggests people speak with a travel vaccine expert about Chikungunya immunization options in 2025.

As the world is confronted with an increasing number of countries reporting polio cases and poliovirus detections, an innovative vaccination strategy has been launched in polio hot spots.
According to the Global Polio Eradication Initiative, the Islamic Republic of Pakistan reported six WPV1 cases in March 2025. Last year, there were 74 polio cases, primarily in Balochistan, Punjab, Khyber Pakhtunkhwa, and Sindh.
To reduce this outbreak, polio vaccination campaigns are deploying innovative delivery solutions.
On March 13, 2025, PharmaJet® announced that their Tropis intradermal (ID) delivery system was used in Pakistan's latest World Health Organization (WHO) polio eradication campaign in February 2025.
Tropis is the first and only needle-free ID delivery technology to achieve WHO prequalification.
Tropis was used to deliver a fractional dose of inactivated polio vaccine (fIPV) in parallel to oral polio vaccine (OPV) administration as part of a WHO-recommended strategy to boost humoral immunity.
Previous data from poliovirus campaigns and routine immunizations in Pakistan, Somalia, and Nigeria have demonstrated that Tropis delivery can improve coverage, decrease cost, and increase acceptability. The data also showed that Tropis is an effective and preferred solution for polio immunization campaigns that can help increase campaign coverage by over 18%.
Also, another study found the potential for up to a 47% decrease in total immunization costs, and 95% of healthcare workers preferred Tropis compared with the SoC.
Paul LaBarre, Vice President of Global Business Development at PharmaJet, commented in a press release, "The Tropis needle-free system is very effective and affordable in campaigns and routine immunizations, protecting nearly 12 million children against poliovirus."
"There is still a lot of work to do, and we are committed to achieving eradication goals so that no child suffers from paralytic polio."
In early 2025, the U.S. CDC reissued a Travel Health Notice regarding polio outbreaks and poliovirus detections in 39 countries to alert travelers of the risks.
In the United States, IPVs have been used to protect people from polio since 2000; OPVs are unavailable in the country.
The CDC recommends that international travelers planning to visit a polio-endemic area speak with a travel vaccine expert about immunization options at least one month before departure abroad.

Over the past decade, vaccine manufacturers have established production centers closer to where the vaccines are utilized, such as in Africa and Europe.
Today, Batavia Biosciences announced a strategic collaboration with Vaccine Biotechnology City (VBC) and MEVAC to enhance vaccine manufacturing capabilities in Egypt.
As of March 13, 2025, this new collaboration builds on a previously signed license agreement for Measles and Rubella (MR) vaccines. Negotiations for Rotavirus vaccines are underway.
This vaccine production capability is essential during disease outbreaks like measles.
Through this partnership, Batavia will license proprietary processes, analytical methods, and production systems to VBC and MEVAC, empowering them to produce high-quality vaccines locally and meet international standards.
This partnership positions Egypt as a vital player in vaccine manufacturing, contributing to greater vaccine self-reliance for the Middle East and Africa.
In a press release, Ahd Hamidi, Strategic Partnership Director at Batavia Biosciences, stated, “This collaboration reflects our mission to deliver affordable and accessible vaccine solutions to regions where they are most needed. By enabling local vaccine production, we aim to empower Egypt as a critical player in the fight against preventable diseases in Africa and beyond.”
As the collaboration progresses, the Batavia, VBC, and MEVAC partnership is set to make a lasting impact on public health outcomes across Africa and the Middle East.

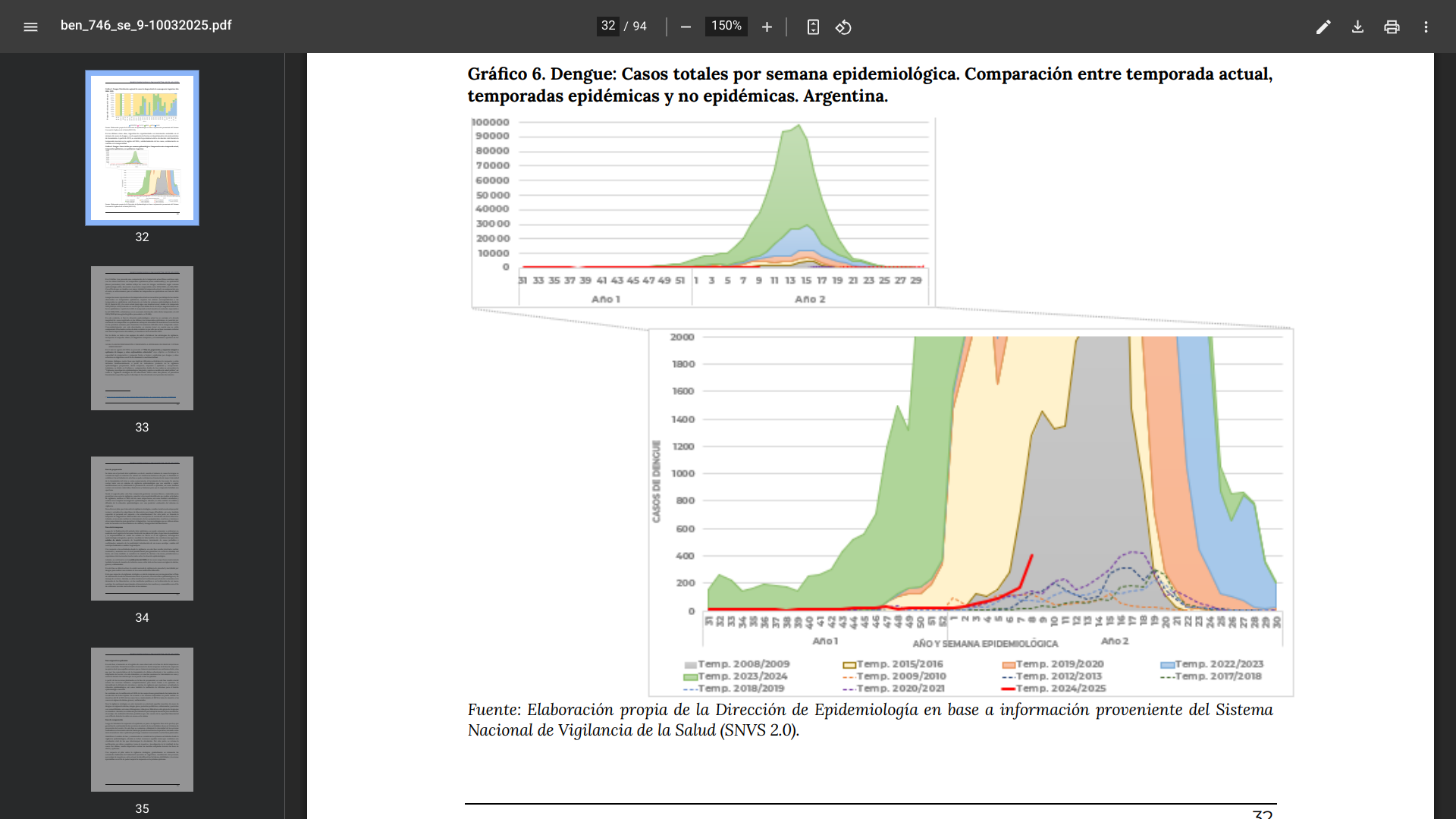
Since the start of the 2024/2025 dengue season in the Argentine Republic during week #31 of 2024, 1,443 cases have been confirmed by the Ministry of Health.
As of March 10, 2025, 96.9% of these dengue cases had no travel history, indicating substantial local mosquito transmission.
Argentina's Central (816) and Northwest (237) regions have reported the most cases this season.
There have been 45 cases with a travel history corresponding to people who traveled to Brazil, Cuba, Mexico, the Maldives, Thailand, India, Peru, Paraguay, and Colombia.
Likewise, millions of international travelers arrived in Argentina last year, most visiting Buenos Aires.
In 2024, about 581,000 dengue cases and 400 related fatalities were reported in Argentina.
As part of the Strategic Plan for Dengue Prevention and Control 2024-2025, Argentina's Minister of Health, Mario Lugones, chaired a meeting on March 11, 2025, regarding actions being carried out, the results achieved to date, and the lines of work to be followed to support and strengthen the jurisdictions' response capacity to address future dengue outbreaks,
Progress was also shared on developing a new national dynamic map that will include geographic, demographic, and climatic data linked to rainfall and temperature records across the country. This will allow for the adoption of preventive measures and the anticipation of potential dengue outbreaks.
To alert international visitors of the health risk, the U.S. CDC reissued a Travel Helath Advisory for the dengue epidemic in the Americas. Since dengue vaccines are currently unavailable in the U.S., the CDC recommends avoiding mosquito bites for travelers to endemic areas.
Additionally, Argentina's Zika virus outbreak has accelerated in 2025, now recording 288 cases this year.
The CDC does recommend various routine and travel vaccinations, such as for chikungunya and yellow fever, before visiting Argentina in 2025.
The European Centre for Disease Prevention and Control (ECDC) released data today that indicates the ongoing transmission of measles across the EU/EEA has continued into 2025.
In 2024, 127,350 measles cases were reported in the European Region, double the cases reported for 2023.
During this period, Romania reported the most cases.
Among those diagnosed with measles with information available on their vaccination status, about 86% were unvaccinated as of March 11, 2025.
This unfortunate disclosure heightens the health risk in Bucharest, Romania's capital city and popular tourism destination. About 6 million people visited Bucharest last year.
In 2024, Romania reported the highest number of measles cases in the European Region, with over 30,000 cases.
According to previous research published in January 2025, Romania's current measles epidemic is driven mainly by two D8 genotype variants with different mutation profiles and slightly different severities.
Phylogenetic analysis identified two well-supported clusters, suggesting at least two local transmission networks in Romania.
These researchers wrote, "To improve vaccination programs in Romania, sustained genetic surveillance of this pathogen and immune waning evaluation of vaccinated adults are recommended."
"The ability to accurately track viral lineages via genetic sequencing is yet another valuable tool for developing an adequate monitoring and surveillance capacity, which can lead to a better identification of transmission chains and, therefore, aid in interrupting measles outbreaks."
Since measles transmission and outbreaks are being reported globally, the ECDC encourages international travellers to check their vaccination status before departing abroad, as the MMR vaccine takes at least two weeks to become effective.
Note: This news article was updated on 3/13/25 with data from the WHO.


