Search API
The World Health Organization (WHO) is hosting a free webinar today titled: Chikungunya: Experiences from the current response to outbreaks in the Americas.
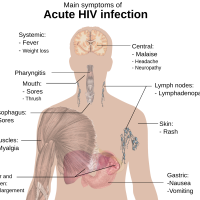
Chikungunya is a viral disease transmitted to humans through the bites of Aedes mosquitoes infected with the chikungunya virus. It has been identified in nearly 115 countries in all the continents except Antarctica.
There are chikungunya vaccine candidates in development as of May 3, 2023. However, there are no approved vaccines.
On May 3, 2023, the speakers were:
- Dr. Sylvie Briand, Director, Epidemic and Pandemic Preparedness and Prevention (EPP), WHO
- Dr. Maria Van Kerkhove, Unit Head, Emerging Diseases and Zoonoses Unit, WHO
- Dr. Thomas Scott, Distinguished Professor of mosquito-transmitted disease ecology and epidemiology, University of California, USA
- Dr. Diana Rojas Alvarez, Technical Lead - Zika and Chikungunya, WHO
- Ms. Thais dos Santos, Advisor on Surveillance and Control of Arboviral Diseases, WHO PAHO
This session can be watched on YouTude.
Vir Biotechnology, Inc. today announced that it received a new $10 million grant from the Bill & Melinda Gates Foundation to support the Phase 1 clinical development of VIR-1388, an investigational novel T cell vaccine for the prevention of human immunodeficiency virus (HIV).
VIR-1388 is based on the human cytomegalovirus vector platform and is designed to elicit T cells that recognize different HIV epitopes with the goal of creating a safe and effective HIV vaccine.
Through close scientific partnerships with the National Institute of Allergy and Infectious Diseases (NIAID) and the HIV Vaccine Trials Network (HVTN), the Phase 1 trial of VIR-1388 (HVTN 142) is expected to begin in the second half of 2023.
The trial will also be funded in part by NIAID, part of the National Institutes of Health, through grant funding to HVTN. In addition, NIAID has provided funding throughout the product development lifecycle of VIR-1388.
Rajesh Gupta, M.D., M.S., M.P.H., Vice President, Global Health Portfolio and Public-Private Partnerships at Vir Biotechnology, stated in a press release on May 2, 2023, “This new grant underscores the importance of our goal of developing innovative solutions for the prevention and treatment of global infectious diseases, including HIV. We look forward to advancing VIR-1388 into the clinic later this year.”
Vir’s support from the Bill & Melinda Gates Foundation includes existing equity investments and grants for the development of therapies for the prevention and treatment of HIV, the prevention of tuberculosis, and the prevention of malaria.
Other HIV vaccine development news is posted by Precision Vaccinations.

Blue Water Vaccines (VWV) Inc. recently announced the signing of a Sponsored Research Agreement with The University of Texas Health Science Center at San Antonio to fund a non-human primate ("NHP") study to evaluate the efficacy of BWV-401, a live attenuated, orally delivered Chlamydia vaccine candidate.
In this new effort, BWV will fund an NHP study to evaluate the efficacy of BWV-401 further and provide additional support for development towards human clinical trials.
In this upcoming study, NHPs will be vaccinated with BWV-401 and subsequently challenged against Chlamydia to validate the hypothesis that, along with being safe, this vaccine, when delivered orally, can elicit an effective immune response in the genital tract and can protect against Chlamydia infection.
BWV-401 utilizes a modified strain of Chlamydia to colonize in the gastrointestinal tract and has produced transmucosal protection against genital tract Chlamydia infection in mouse models without altering the gut microbiota.
"We are thrilled to initiate this study with our partners at UT Health Science Center San Antonio for BWV-401," said Joseph Hernandez, Chairman and Chief Executive Officer of BWV, in a press release on April 12, 2023.
"There remains a high unmet need for an efficacious Chlamydia vaccine to prevent the millions of infections worldwide each year."
According to the U.S. CDC, Chlamydia is the most frequently reported bacterial sexually transmitted infection in the U.S., with about 1.6 million new cases reported in 2020.
As of April 30, 2023, no U.S. FDA-approved Chlamydia vaccines.

In the United States, there remains a considerable burden of disease attributed to serotypes not included in currently approved pneumococcal conjugate vaccines.
To address this need, Pfizer Inc. today announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20® for the prevention of invasive pneumococcal disease (IPD) caused by the 20 Streptococcus pneumoniae serotypes contained in the vaccine in infants and children six weeks through 17 years of age.
And for preventing otitis media in infants six weeks through five years of age caused by the original seven serotypes contained in PREVNAR®.
"Today's FDA approval of our vaccine, PREVNAR 20, now offers parents the ability to help protect their children against 20 pneumococcal serotypes in circulation, which represent the majority of pneumococcal disease in U.S. infants and children," said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on April 27, 2023.
"This important PREVNAR 20 approval builds on more than 20 years of real-world impact with PREVNAR and PREVNAR 13, safety data, and effectiveness, highlighting Pfizer's leadership in developing groundbreaking pneumococcal conjugate vaccines to help protect infants and their families from life-threatening infections."
Pneumococcal vaccine news is posted by Precision Vaccinations.

ImmunityBio, Inc. today announced the opening of a clinical trial to study its investigational Tri-Ad5 vaccine combination and its IL-15 superagonist N-803 for people with a hereditary condition known as Lynch syndrome.
An estimated 1 in every 300 people may be carriers of a mutation in a gene associated with Lynch syndrome.
This Phase 2b clinical trial, sponsored by the National Cancer Institute (NCI), will study whether Tri-Ad5, in combination with N-803, works to prevent colorectal and other cancers in study participants.
Tri-Ad5 vaccines target three tumor-associated antigens: brachyury, carcinoembryonic antigen, and mucin-1.
The vaccine combination studies whether activating dendritic cells and training the immune system to recognize those proteins will destroy the precancer cells before the cancer advances.
N-803 is designed to enhance the effects of the vaccines by increasing the proliferation and activation of natural killer and T cells, thereby increasing the potential for cancer prevention in study participants.
"We are excited to partner with the NCI on this important cancer vaccine study to potentially prevent or delay the onset of cancer for people who carry the gene associated with Lynch syndrome," said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on April 25, 2023.
"Lynch syndrome affects tens of thousands of people each year, and the average age of cancer diagnosis for them is just 44."
"People known to have the gene for Lynch syndrome can be followed closely by their doctors with regular examinations and scans to watch for cancer development, but, currently, there is no treatment that prevents cancer development in these patients."
Lynch syndrome is one of the most common hereditary cancer syndromes, says the U.S. CDC.
Not only can people with Lynch syndrome develop colorectal cancer some twenty years before the average age of diagnosis for this cancer, but they are also at an increased risk of developing multiple types of other cancers, including endometrial, stomach, ovarian, pancreas, ureter, and renal pelvis, biliary tract, brain, and small intestinal cancers.
Colorectal cancer is the second-deadliest cancer type in the U.S., and approximately 3% to 5% of the 153,000 cases of colorectal cancer annually are thought to be due to Lynch Syndrome.
As are 2% to 3% of all cases of endometrial cancer.
"The Tri-Ad5 vaccine trial will be the largest Lynch syndrome cancer prevention study done in the U.S.," commented Asad Umar, D.V.M., Ph.D., a senior advisor to the director for translational research in NCI's Division of Cancer Prevention and a scientific lead for the trial.
Note: ImmunityBio is also conducting a bladder cancer vaccine study.

Global health partners today announced a call for "The Big Catch-up," a targeted effort to boost vaccination among children following declines driven by the COVID-19 pandemic.
The Big Catch-up aims to protect populations from vaccine-preventable outbreaks, save children's lives, and strengthen national health systems.
With over 25 million children missing at least one vaccination in 2021, outbreaks of preventable diseases, including measles, diphtheria, polio, and yellow fever, are already becoming more prevalent and severe.
The WHO, UNICEF, Gavi, the Vaccine Alliance, the Bill & Melinda Gates Foundation, and Immunization Agenda 2030 aim to reverse the declines in childhood vaccination recorded in over 100 countries since the pandemic.
The 20 countries where three-quarters of the children who missed vaccinations live are Afghanistan, Angola, Brazil, Cameroon, Chad, DPRK, DRC, Ethiopia, India, Indonesia, Nigeria, Pakistan, Philippines, Somalia, Madagascar, Mexico, Mozambique, Myanmar, Tanzania, Viet Nam.
"Vaccines are a public health triumph," commented Dr. Chris Elias, president of Global Development at the Bill & Melinda Gates Foundation, in a press release on April 24, 2023.
"The incredible progress that has been made toward ending polio and reducing the incidence of infectious diseases is the direct result of thousands of dedicated global partners and local health workers who have worked to immunize millions of children."
"We must double down to reach all children with the vaccines they need to live healthier lives and ensure that future generations live free of preventable diseases like polio."