Search API
The journal Nature Medicine recently asked researchers to name their top clinical trial picks for 2024, from base editing and a vaccine against human immunodeficiency virus (HIV).
On December 7, 2023, Carrie Arnold and Paul Webster wrote ...with so many rollercoaster years since the start of the pandemic, it is impossible to predict exactly what the biomedical world will deliver in 2024.
Experts identified which trials will likely have an outsized impact on medicine in 2024.
One expert, Carey Hwang, a senior vice president and head of clinical research at Vir Biotechnologycommented, highlighted VIR-1388, a cytomegalovirus (CMV) vector vaccine that induces strong, unique, and sustained T cell responses that can potentially prevent the acquisition of HIV.
The HIV Vaccine Trials Network is conducting a clinical trial at ten sites in the U.S. and two sites in South Africa, with support from the U.S. National Institute of Allergy and Infectious Diseases and the Bill & Melinda Gates Foundation.
From a public health perspective, having a vaccine against HIV would have a tremendous impact, commented Hwang.
As of December 8, 2023, the U.S. FDA has not approved any HIV vaccine candidates.
One of the world’s most formidable public health challenges, human immunodeficiency virus (HIV), is impacting more than 38 million people worldwide.
While there are no U.S. Food and Drug Administration (FDA) approved HIV vaccines in 2023, a novel arenaviral therapeutic vaccine candidate is being evaluated as a potential curative regimen for HIV.
HOOKIPA Pharma Inc. today announced that it has received clearance from the FDA for its Investigational New Drug (IND) application for HB-500, a novel arenaviral therapeutic vaccine for the treatment of HIV.
HB-500 is an alternating, 2-vector arenaviral therapeutic vaccine for the treatment of HIV.
One vector is based on the lymphocytic choriomeningitis virus as its arenaviral backbone; another vector is based on the Pichinde virus.
Both encode the same HIV antigens. The alternating 2-vector approach is designed to further focus the immune response against the target antigen.
HOOKIPA is responsible for advancing the HIV program through the completion of a Phase 1b clinical trial.
And Gilead Sciences, Inc. has the exclusive right to assume further program development afterward.
Joern Aldag, Chief Executive Officer at HOOKIPA, commented in a press release on November 20, 2023, “The ability to generate a potent and broad T cell response that can clear infected cells is critical for HIV control. Our novel arenaviral therapeutic vaccine (candidate) offers promise in helping to address the unmet need for a functional HIV cure.”
In November 2023, Nature Partner Journals Vaccines published the joint-preclinical research by HOOKIPA and Gilead, which served as the foundation for the IND submission.
The data show that arenaviral therapeutic vaccination was well tolerated and generated robust, high-quality, and durable immune responses (antigen-specific T cells and antibodies) in non-human primates.
Additionally, arenaviral therapeutic vaccination significantly reduced SIV viral load and clinical illness in those animals compared to placebo.
HIV, a sexually transmitted disease, is one of the world’s most formidable public health challenges.
The virus infects and kills immune cells and, without effective ongoing treatment, leaves the individual increasingly immunocompromised over time. While effective treatments have significantly extended the lives of people living with HIV and reduced the transmission of the virus, there is no cure for HIV or AIDS in 2023.
Precedence Statistics today announced the global virology market size reached $2.6 billion in 2022 and is projected to reach $4.26 billion by 2032, expanding at a CAGR of 5.10%.
The U.S. virology market reached $690 million in 2022 and is projected to expand at a CAGR of 5.20%, reaching around $1.14 billion by 2032.
The virology market encompasses the study, diagnosis, treatment, and prevention of viral infections. It includes research, pharmaceuticals, diagnostic tests, and vaccines related to viruses like HIV, influenza, and hepatitis, which is a significant driver for the growth of the virology market.
The rapid mutation rates of many viruses pose hurdles for drug and vaccine development, necessitating ongoing research and adaptability.
The quest for effective vaccines to prevent viral diseases has also driven growth in this market. Government investments and public health initiatives have played a pivotal role in shaping the industry landscape.
The continuous evolution of vaccine technology, including mRNA and vector-based platforms, has broadened the scope of virology research.
Furthermore, the need for vaccine booster shots to combat emerging virus variants ensures a sustained demand for virology products.
Furthermore, the resumption of global travel contributes to the rapid spread of viruses, emphasizing the importance of virology in understanding, preventing, and managing infectious diseases worldwide.

A study led by Chicago Department of Public Health researchers published in the Journal of Infectious Diseases involved estimating rates of HIV, gonorrhea, and chlamydia among mpox patients.
This study was published on November 8, 2023, and identified factors related to mpox severity from June 2022 to March 2023.
These researchers concluded that sexually transmitted infections (STIs) could facilitate mpox transmission.
Of the 1,124 mpox patients, 44% had HIV, and 70% had a previous or current STI, with 39% having had at least three previous STI episodes.
Of 335 vaccinated mpox patients, 55% had received one dose of the JYNNEOS® (MVA-BN) vaccine, and 45% had received two doses.
In total, 17.6% has received one or more JYNNEOS vaccination before mpox infection.
The U.S. Centers for Disease Control and Prevention (CDC) reported on October 25, 2023, that post-JYNNEOS vaccination reinfection cases have been published and that they are aware of less than 10 cases of probable reinfection.
The CDC reported Vaccine Effectiveness of JYNNEOS against mpox ranges from 36%–75% for 1-dose vaccination and 66%–89% for 2-dose vaccination.
"Future research should examine predictors of mpox infection among those with STIs, including other STIs, such as syphilis, HIV risk at STI screening or anatomical site of infection," wrote these researchers.
As of October 2023, there has been a significant increase in mpox outbreaks in the European Region.
In the last month, 21 countries reported mpox cases, with Portugal reporting the highest relative increase in cases (n = 86).

The World Health Organization's (WHO) 2023 Global Tuberculosis (TB) report, announced today, shows the impact of this centuries-old disease.
The report, published on November 7, 2023, features TB outbreak data from 192 countries and areas and shows that 7.5 million people were diagnosed in 2022, the highest figure recorded since 1995.
According to the WHO, an estimated 10.6 million people fell ill with TB in 2022, up from 10.3 million in 2021.
And the total number of TB-related deaths (including those among people with HIV) was 1.3 million in 2022. TB continues to be the leading killer among people with HIV.
Geographically, most people who developed TB in 2022 were in South-East Asia (46%), Africa (23%), and the Western Pacific (18%), with smaller proportions in the Eastern Mediterranean (8.1%), the Americas (3.1%) and Europe (2.2%).
In a press release, Dr. Tereza Kasaeva, Director of WHO's Global TB Programme, commented, "This report provides key data and evidence on the status of the TB epidemic and a review of the progress that serves to inform the translation of these targets and commitments into action in countries."
"We need all hands on deck to make the vision of ending TB a reality."
TB is a vaccine-preventable disease, with about 16 different Bacille Calmette-Guérin (BCG) vaccines in use globally.
In the U.S., access to the BCG vaccine is limited and considered for people who meet specific criteria. Merck's TICE® BCG vaccine is an attenuated, live culture preparation of the BCG strain of Mycobacterium Bovis and is available in 2023.

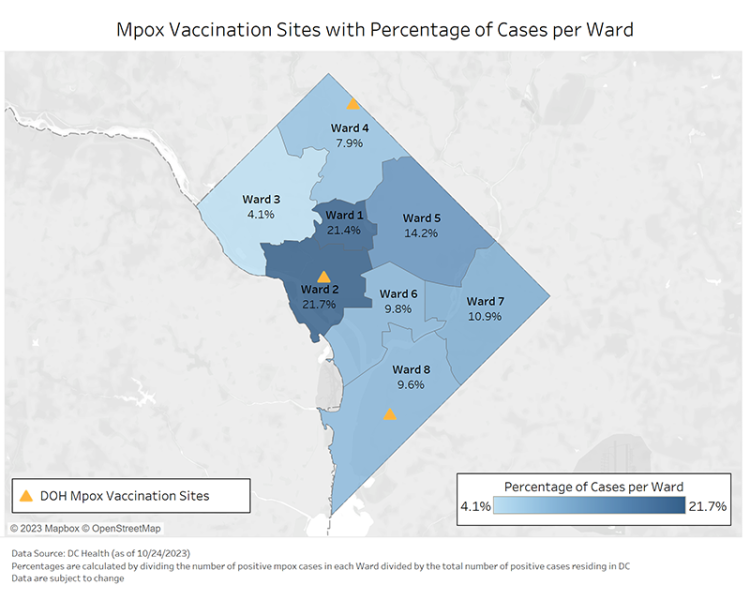
According to a study published in Sexually Transmitted Diseases, most study participants receiving mpox vaccination in Washington, DC, reported decreasing sexual behaviors associated with mpox virus transmission.
Overall, between 46%–61% of study participants reported a decrease in sexual behaviors associated with mpox.
In DC, over 41,000 mpox vaccinations have been administered since the global outbreak began in May 2022.
Published on October 26, 2023, the study was led by U.S. Centers for Disease Control and Prevention researchers and involved 711 adults seeking mpox vaccination from August to October 2022.
The median participant age was 32 years; 52% were White, 20.5% were Black, 14.6% were Hispanic, 7.9% were Asian, 2.0% were multiracial, and 0.3% were American Indian/Alaska Native.
And 9% had HIV.
Most of the study participants were men who had sex with men (61%), 27% were women, and 3.8% were men who had sex with only women.
According to D.C. Health, there have been 543 cumulative mpox cases, 24 hospitalizations, and 0 related fatalities since May 2022.
Mpox is a sexually transmitted disease that is often prevented with a U.S. FDA-approved vaccine.
Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®) mpox-smallpox vaccine was offered in the U.S. during this study.
A study published by The Lancet Infectious Disease on October 11, 2023, attempted to answer questions regarding the durability and strength of protection following infection and JYNNEOS vaccination.
This analysis reported that people vaccinated with JYNNEOS frequently developed low or medium mpox-neutralizing antibodies compared to infected individuals.
As of October 31, 2023, the JYNNEOS vaccine remains available in key cities in the U.S.
Bavarian Nordic A/S today announced the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted in favor of recommending the routine use of JYNNEOS® (MVA-BN®), the Company’s U.S. FDA-approved mpox / smallpox vaccine.
Specifically, the ACIP voted to recommend that individuals 18 years and older with certain risk factors receive the two-dose JYNNEOS regimen, a live, nonreplicating vaccine.
Previously, the ACIP had recommended JYNNEOS for individuals at risk during a mpox outbreak.
This represents the second national recommendation for Bavarian Nordic’s mpox vaccine in adult risk groups following a similar endorsement by the Standing Committee for Vaccination in Germany in 2022.
More recently, the European AIDS Clinical Society also recommended using the vaccine for adults infected with HIV or on pre-exposure prophylaxis treatment, which may support additional national recommendations for future vaccine use.
“Since the outbreak of mpox last year (May 2022), Bavarian Nordic has supplied millions of doses of our vaccine to more than 70 countries worldwide,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on October 25, 2023.
The CDC estimates that 2 million U.S. individuals are eligible for a vaccination against mpox under this recommendation.
To date, approximately 23% of this group has received the recommended two doses of JYNNEOS, leaving a significant number of people vulnerable to infection with mpox.
The ACIP reported in October 2023 JYNNEOS Vaccine Effectiveness against mpox ranges from 36% to 75% for 1-dose vaccination and 66% to 89% for 2-dose vaccination.
Pending approval of the updated recommendations, Bavarian Nordic is targeting a commercial launch of JYNNEOS in the U.S. in the first half of 2024.



