Search API
GSK plc today announced the U.S. Food and Drug Administration (FDA) had granted a Fast Track designation for its Neisseria gonorrhoeae investigational vaccine (NgG).
Fast Track designation is intended to facilitate the development and expedite the review of potentially important new drugs and vaccines to treat or prevent serious conditions with unmet medical needs.
As of June 27, 2023, the vaccine candidate is conducting a Phase II clinical trial and aims to demonstrate proof of concept by assessing the efficacy of the NgG vaccine in healthy adults.
Phil Dormitzer, Global Head of Vaccines R&D, GSK, commented in a related press release, "This designation recognizes the potential for a vaccine that could help protect millions of people across the world against the serious health consequences of infection with a bacterium that is considered a 'high priority' pathogen by the World Health Organisation."
Gonorrhoea is the second most prevalent bacterial sexually transmitted infection worldwide, with an estimated 82 million new cases yearly.
In the U.S., rates of reported gonorrhea have increased by 118% from 2009 to 2021.
Furthermore, antimicrobial resistance to gonorrhea has increased over the past 80 years, rendering many classes of antibiotics used to treat the disease ineffective.
Vaccines can play a critical role in the fight against AMR by helping prevent bacterial, viral, and other infections.
Currently, no gonorrhea-specific vaccines are approved anywhere in the world, says GSK.
However, in France, the meningococcal (MenB-4C) vaccine is recommended against gonorrhea.
And Intravacc's Avacc 11® is the prophylactic intranasal gonorrhea candidate vaccine.
As of June 28, 2023, gonorrhea vaccine and treatment news have been published by Precision Vaccinations.

IAVI announced today that the initial participants had been vaccinated with a Sudan virus (SUDV) vaccine candidate in a first-in-human Phase I clinical trial in the U.S.
As of June 27, 2023, the IAVI C108 IAVI-sponsored trial is funded by the Biomedical Advanced Research and Development Authority (BARDA).
IAVI C108 will occur at two U.S.-based clinical trial sites, where the vaccine candidate will be administered intramuscularly at three dosage levels.
This is essential news since there are no SUDV vaccines available.
Furthermore, like the Zaire Ebolavirus (ZEBOV), SUDV is responsible for recurring viral hemorrhagic fever outbreaks across sub-Saharan Africa.
In past Ebola outbreaks, the estimated case fatality ratios of SUDV disease have varied from 41% to 100%.
This study evaluates the safety and immunogenicity of an investigational SUDV vaccine candidate previously donated to IAVI by Merck. This investigational SUDV vaccine candidate was produced for IAVI from an existing investigational bulk drug substance previously manufactured by Merck.
IAVI is responsible for all aspects of the candidate’s future development, including demonstrating equivalence between this SUDV vaccine candidate and IAVI’s other SUDV vaccine candidate, which utilizes the same viral vector but is manufactured using a new production platform.
The SUDV vaccine candidate being evaluated in IAVI C108 uses the same recombinant vesicular stomatitis virus (rVSV) viral vector platform as ERVEBO®, Merck’s single-dose ZEBOV vaccine, which is licensed in the U.S., U.K., European Union, Canada, Switzerland, and 10 African countries.
“IAVI C108 represents an important first step toward generating the data needed for eventual licensure of an rVSV-SUDV vaccine. The development and licensure of ERVEBO® have resulted in an important tool in Ebola Zaire outbreak responses. If proven effective, we’re hopeful that a vaccine candidate built on the same viral platform will be similarly important in future SUDV outbreaks,” said Swati Gupta, Ph.D., vice president and head of emerging infectious diseases and epidemiology at IAVI, in a related press release.
The rVSV platform has been used extensively in adults and children. The underlying vesicular stomatitis virus is a common animal virus that does not cause serious illness in humans and has been investigated extensively as a vaccine vector.
In the vaccine platform, it is engineered to encode a surface protein from a target pathogen, in this case, SUDV, to prompt the body to mount an immune response.
Much of the research and development on IAVI’s rVSV platform is performed at the IAVI Vaccine Design and Development Lab in Brooklyn, New York.

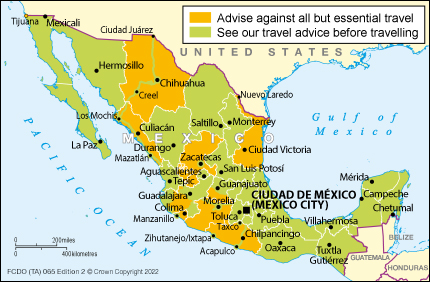
The U.K. The Foreign, Commonwealth, and Development Office (FCDO) recently advised against all but essential travel to various states in Mexico as some areas of Mexico have a high crime rate and civil unrest.
On June 22, 2023, the FCDO stated that when considering travel to any of these areas in Mexico, including Cancun, please see the Safety and Security section for more detailed information on the risks.
Additionally, if you plan to pass through another country to return to the U.K., check the travel advice for your transiting country says the FCDO.
From a health perspective, the U.S. CDC issued a Disease Outbreak News, confirming an outbreak of suspected fungal meningitis associated with surgical procedures performed under spinal anesthesia.
The CDC suggests speaking with a healthcare provider about travel vaccine options, such as dengue, one month before visiting Mexico.
Sanofi - Aventis Groupe today announced positive topline Phase 2b clinical data in atopic dermatitis support amlitelimab as a potential first and best-in-class novel investigational anti-OX40-ligand monoclonal antibody.
The primary endpoint was met in the Phase 2b study (STREAM-AD) of amlitelimab in adults with moderate-to-severe atopic dermatitis whose disease cannot be adequately controlled with topical medications or for whom topical medications are not a recommended treatment approach.
Amlitelimab is a fully human non-depleting monoclonal antibody that binds to OX40-Ligand, a key immune regulator.
It can be a first-in-class treatment for various immune-mediated diseases and inflammatory disorders, including moderate-to-severe atopic dermatitis and asthma.
By targeting OX40-Ligand, amlitelimab aims to restore immune homeostasis between pro-inflammatory and regulatory T cells.
Naimish Patel, M.D., Head of Global Development, Immunology and Inflammation, Sanofi, commented in a press release on June 27, 2023, "While we have made significant strides in the treatment of atopic dermatitis, there are patients who are still in need of new options."
"We believe that the results from this Phase 2b study with amlitelimab support our perspective that targeting OX40-Ligand has the potential to provide a first and best-in-class treatment option that addresses type 2 and non-type 2 inflammation to meet the individual needs of people living with atopic dermatitis and other chronic inflammatory diseases."
"We look forward to advancing into a larger Phase 3 clinical development program and continuing to drive momentum in our Immunology pipeline to deliver first or best-in-class treatments."
In this dose-ranging study, treatment with amlitelimab resulted in statistically significant improvements in average Eczema Area and Severity Index score from baseline at 16 weeks compared to placebo for all four subcutaneous doses that were studied.
There were also improvements in key secondary outcome measures, and continued improvements were observed through week 24 in primary and key secondary outcomes.
Biomarker results support an effect on both type 2 and non-type 2 pathways.
Amlitelimab was well-tolerated in the study across all dose arms, and no new safety concerns were identified.
Furthermore, Amlitelimab is under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
The U.S. NIH says atopic dermatitis, often referred to as eczema, is a chronic (long-lasting) disease that causes inflammation, redness, and irritation of the skin. It is a common condition that usually begins in childhood.
However, anyone can get the disease at any age.
And atopic dermatitis is not contagious, so it cannot be spread from person to person.

AC Immune SA today announced that it had received Fast Track designation from the U.S. Food and Drug Administration (FDA) for its anti-amyloid beta (Abeta) active immunotherapy (vaccine)-candidate, ACI-24.060, for the treatment of Alzheimer’s disease (AD).
Confirmed on June 27, 2023, this news follows FDA clearance of the Investigational New Drug (IND) application enabling expansion to the USA of the ongoing Phase 1b/2 ABATE study of ACI-24.060 in patients with AD and individuals with Down syndrome (DS).
Furthermore, the first individual with DS has been dosed in ABATE.
Dr. Michael Rafii, Medical Director of the Alzheimer’s Therapeutic Research Institute, Professor of Neurology at the Keck School of Medicine, and the Principal Investigator of the clinical trial, commented in a press release, “Despite representing the world’s largest population that is genetically at high risk for AD, individuals with DS are vastly underserved and underrepresented in clinical trials."
"I applaud AC Immune for seeking to address the urgent needs of this population and believe ACI-24.060 holds great promise as a novel therapy that can lower Abeta plaques to delay, or perhaps even prevent, the onset of clinical dementia symptoms in AD and DS-related AD."
"Moreover, I believe the potential safety, efficacy, and logistical advantages of a vaccine over monoclonal antibodies strongly support the development of therapeutics such as ACI-24.060 as the next generation of anti-Abeta therapies.”
Alzheimer's vaccine candidates are not FDA-approved as of June 27, 2023.

The journal Nature Communications recently published an article that concluded interim data from 2 parts of the phase 1/2 clinical trial support the continued development of mRNA-1010.
The mRNA-1010 vaccine candidate elicited either higher or comparable immune responses to a standard-dose, influenza quadrivalent inactivated vaccine.
Overall, these first-in-human safety and immunogenicity findings highlight, on June 19, 2023, the potential of the mRNA platform to improve the effectiveness of influenza vaccines.
Vaccines using mRNA technology are readily amenable to antigenic drift and shift in influenza strains, allowing for rapid deployment of vaccines. In addition, mRNA-based platforms allow for the expression of multiple antigens, raising the possibility for an increased breadth of protective responses against seasonal influenza or multiple respiratory diseases.
However, approved cell-based influenza vaccines, such as Flucelvax® Quadrivalent (QIVc), currently produce an exact antigenic match for circulating flu trains.
Further, based on findings with mRNA-1273, an mRNA-based vaccine against SARS-CoV-2, mRNA vaccines may also induce strong cellular immune responses and prolonged germinal center reactions that can improve protection in older adults, a population at particular risk for infection and severe outcomes.
While mRNA-1010 had an acceptable safety profile in this trial, transient solicited adverse reactions were more common after mRNA-1010 than with the active comparator.
Additional clinical trials are ongoing to assess further this vaccine candidate's safety, efficacy, and immunogenicity and a licensed influenza vaccine comparator (NCT05566639 and NCT05415462).
Moderna, Inc. was involved in the study design, data collection and analysis, and the writing of this manuscript. Moderna funded this study.

Invivyd, Inc. today announced that it has reached general alignment with the U.S. Food and Drug Administration (FDA) on a pathway to potential emergency use authorization (EUA) for VYD222 and anticipated follow-on monoclonal antibody (mAb) candidates designed to prevent symptomatic COVID-19.
The company plans to leverage the pathway, which includes the use of serum-neutralizing titers as a correlate of protection in an immunobridging approach to a pivotal clinical trial of VYD222, to rapidly generate data to support a potential VYD222 EUA for the prevention of symptomatic COVID-19.
Based on FDA feedback, the use of a correlate of protection in an immunobridging approach to a pivotal EUA-directed clinical trial may be a reasonable approach for a new mAb candidate when clinical trial data from a "prototype" mAb is available, provided that the new mAb candidate:
- Is similar to the prototype mAb such that it leverages a consistent manufacturing platform and has limited structural and functional differences, and,
- Has supportive nonclinical data, such as favorable in vitro neutralization data against currently circulating SARS-CoV-2 variants.
"We are very encouraged by the recent feedback from the FDA and appreciate their commitment to exploring alternative strategies to expedite the development of mAbs for the prevention of symptomatic COVID-19, such as the use of a correlate of protection as the primary endpoint in a pivotal clinical trial, a strategy that we are pleased to see further advance following the joint EMA-FDA workshop last December where the approach was discussed," said Dave Hering, chief executive officer of Invivyd, in a press release on June 26, 2023.
"Given our previous work developing adintrevimab and our platform-based approach to rapid mAb discovery, we believe we are one of few companies positioned to rapidly and serially generate data for potential EUA submission for next-generation mAb candidates for the prevention of symptomatic COVID-19."
"This potential pathway supports the company's vision and strategy of establishing a platform and stream of optimized anti-SARS-CoV-2 mAb candidates that can be deployed to keep pace with viral evolution and protect the vulnerable."
COVID-19 antibody and antiviral news was updated on June 26, 2023.
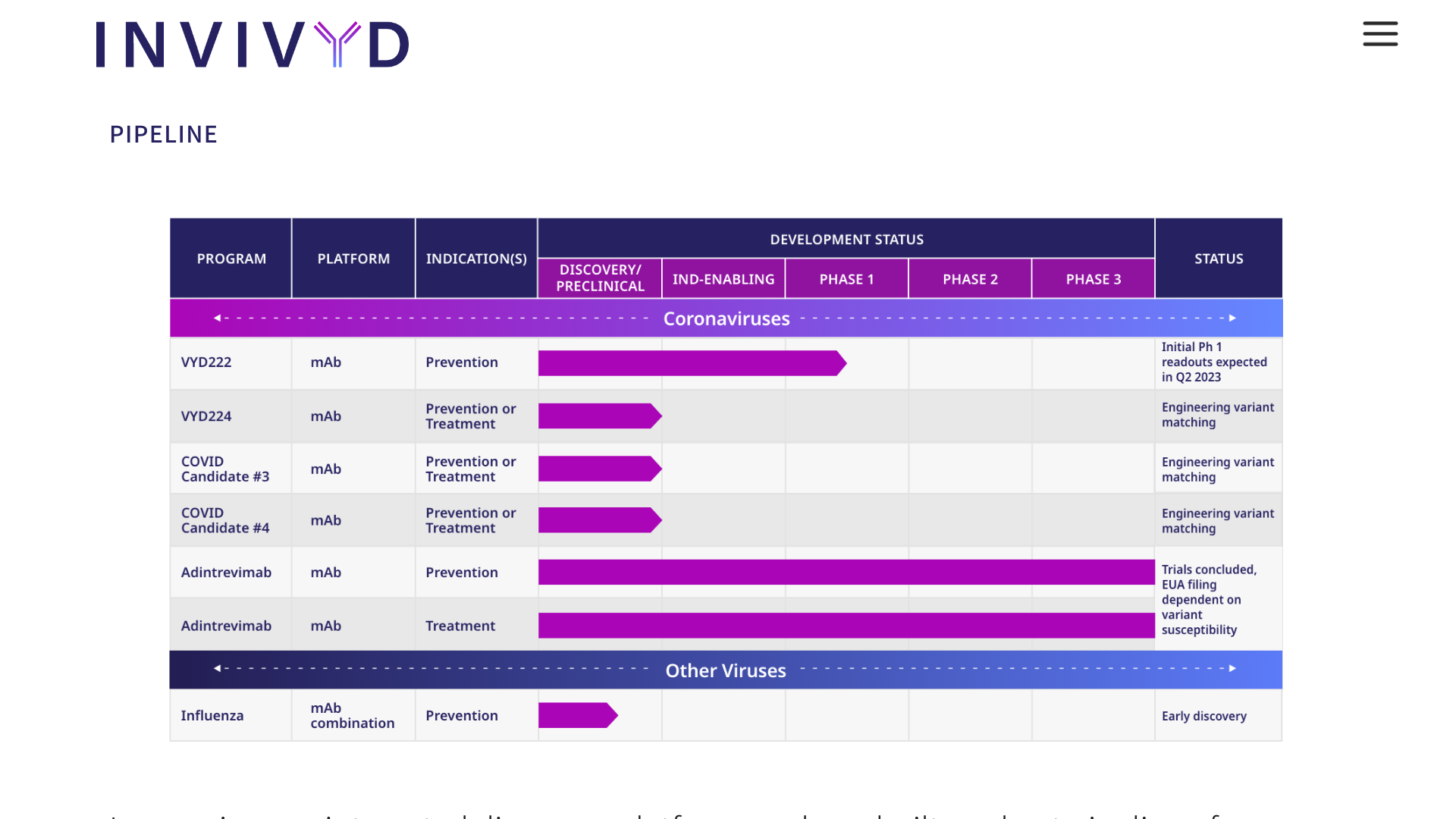
Invivyd, Inc. recently announced positive initial data from its ongoing Phase 1 clinical trial of its lead investigational monoclonal antibody (mAb) candidate, VYD222.
VYD222 is a broadly neutralizing, half-life extended mAb candidate in development for the prevention of symptomatic COVID-19 in vulnerable populations, such as immunocompromised people.
Initial Phase 1 data show that a single administration of VYD222 was generally well-tolerated at all three dose levels tested, with no serious adverse events reported to date.
At the lowest VYD222 dose tested (1500 mg), geometric mean serum neutralizing titers were 3245.1 (95% CI: 1882.5, 5594.0) against Omicron XBB.1.5 at Day 7, with a geometric mean 38.87-fold rise (95% CI: 10.3, 146.8) from baseline to Day 7 (n=8).
Greater VYD222 dose levels are designed to provide greater protection from any potential loss of neutralization activity as SARS-CoV-2 evolves.
Dave Hering, chief executive officer of Invivyd, stated in a press release on June 22, 2023, "Based on previously published clinical data from randomized controlled clinical trials, we believe that mAb directed against the receptor binding domain of the SARS-CoV-2 spike protein offer an attractive safety profile, even at higher doses, and that strong serum neutralization activity would be predictive of clinical benefit."
Additional COVID-19 monoclonal antibody news is posted by Precision Vaccinations.