Search API
Moderna, Inc., a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, and Merck today announced the initiation of the pivotal Phase 3 randomized V940-001 clinical trial evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy (INT), in combination with KEYTRUDA, Merck's anti-PD-1 therapy, as an adjuvant treatment in patients with resected high-risk (Stage IIB-IV) melanoma.
INTs are designed to train and activate the immune system so that a patient can generate an antitumor response specific to their tumor mutation signature.
V940-001 is the first Phase 3 study of a planned comprehensive clinical development program initiated following the positive primary analysis of the Phase 2b KEYNOTE-942/mRNA-4157-P201 trial.
Global recruitment in V940-001 has begun, and the first patients are now enrolling in Australia.
"The initiation of the V940-001 Phase 3 trial is an exciting and important milestone for us as we work with our colleagues at Merck and the melanoma patient community to investigate how individualized neoantigen therapy may potentially transform the treatment of the most serious form of skin cancer," said Kyle Holen, M.D., Moderna's Senior Vice President and Head of Development, Therapeutics and Oncology, in a press release on July 26, 2023.
Melanoma, the most serious form of skin cancer, is characterized by the uncontrolled growth of pigment-producing cells.
Nearly 325,000 new cases were diagnosed worldwide in 2020.
In the U.S., skin cancer is one of the most common types of cancer diagnosed, and melanoma accounts for a large majority of skin cancer deaths. It is estimated there will be nearly 100,000 new cases of melanoma diagnosed and almost 8,000 deaths resulting from the disease in the U.S. in 2023.

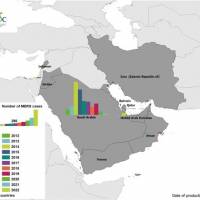
The World Health Organization (WHO) today announced a case of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) cases was confirmed in Abu Dhabi, the United Arab Emirates (UAE).
According to the WHO's Disease Outbreak News on July 24, 2023, this MERS-CoV case had no history of direct or indirect contact with camels, goats, or sheep.
Prior to this WHO notification, the last MERS-CoV infection reported from the UAE was in November 2021.
Since July 2012, the UAE has confirmed 94 MERS-CoV cases and 12 related fatalities.
The WHO expects that additional cases of MERS-CoV infection will be reported from the Middle East.
MERS-CoV cases have reached 2,605 globally, including 936 associated deaths as of July 2023.
These are MERS vaccine candidates in development, but the WHO has not approved any.

Emergent BioSolutions Inc. today announced that the U.S. Food and Drug Administration (FDA) approved CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted) (AV7909) for post-exposure prophylaxis (PEP) of disease following suspected or confirmed exposure to Bacillus anthracis in adults when administered in conjunction with recommended antibacterial drugs.
The efficacy of the CYFENDUS™ vaccine for PEP is based solely on studies in animal models of inhalational anthrax.
Emergent's anthrax franchise includes the BioThrax® vaccine, which will continue to serve a critical purpose, as well as two treatments, Anthrasil®, a polyclonal antibody therapeutic, and raxibacumab, a monoclonal antibody therapeutic.
Dr. Kelly Warfield, Emergent's senior vice president, science and development, commented in a press release on July 20, 2023, "The 20-year journey from early development to approval is a major milestone that attests to Emergent's scientific and technical prowess and partnering capabilities."
"We are grateful for the yearslong collaboration with the Biomedical Advanced Research and Development Authority and early support from the Defense Advanced Research Projects Agency and the National Institute of Allergy and Infectious Diseases.
CYFENDUS vaccine is comprised of Anthrax Vaccine Adsorbed and an additional adjuvant. It has been demonstrated that using an additional adjuvant, two doses administered over 14 days elicit protective levels of an immune response, which can be especially important in response to a large-scale public health emergency involving anthrax.
In December 2018, the CYFENDUS vaccine was the subject of a pre-emergency use authorization package submitted to the FDA. The following year, the U.S. government procured this product for national preparedness efforts.

Pfizer Inc. today announced data from a Phase 2 clinical study investigating its hexavalent capsular polysaccharide (CPS) conjugate Group B Streptococcus (GBS) vaccine candidate, GBS6.
This vaccine candidate is being developed for maternal administration to protect infants against invasive GBS disease.
In stage two of the three-part study, GBS6 generated robust maternal antibody responses against the six GBS CPS serotypes included in the vaccine, and these antibodies were efficiently transferred to infants at ratios of ~0.4-1.3 depending on the GBS6 group.
Based on a parallel natural history study conducted in South Africa, the Phase 2 study immunogenicity data suggest that GBS6 may offer meaningful protection against invasive GBS disease in newborns and young infants.
The results were published in an Original Article in The New England Journal of Medicine on July 20, 2023, and will inform a planned Phase 3 clinical development program.
“Group B Streptococcus can cause potentially devastating diseases in infants, including sepsis, pneumonia, and meningitis. Annually, there are nearly 400,000 cases of infant disease and approximately 138,000 stillbirths and infant deaths worldwide due to GBS,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on July 19, 2023.
“Building on decades of expertise and knowledge in vaccines, we are committed to helping protect newborns and young infants through maternal immunization.”
The U.S. CDC says bacteria called group B Streptococcus cause GBS disease.
GBS bacteria commonly live in people’s gastrointestinal and genital tracts. The genital tract is the part of the body involved in reproduction and includes the vagina in women.
Sometimes the bacteria invade the body and cause certain infections, which are known as GBS disease.

Vir Biotechnology, Inc. today announced that the Phase 2 clinical trial (PENINSULA) evaluating VIR-2482 for preventing symptomatic influenza A illness did not meet primary or secondary efficacy endpoints.
In participants who received the highest dose of the investigational hemagglutinin-targeting monoclonal antibody (mAb) VIR-2482 (1,200 mg), a non-statistically significant reduction of approximately 16% in influenza A protocol-defined illness was observed.
And participants who received the highest dose showed an approximately 57% reduction in symptomatic influenza A illness when defined according to U.S. CDC influenza-like-illness criteria, which was one of two secondary endpoints.
VIR-2482 was generally well tolerated, and no safety signals were identified.
“We are grateful to all who participated in this trial, and we remain committed to the pursuit of novel therapies that have the potential to address some of the world’s most serious infectious diseases,” said Marianne De Backer, M.Sc., Ph.D., MBA, Vir’s Chief Executive Officer, in a press release issued on July 2023.
The PENINSULA trial has been supported in whole or in part with federal funds from the Department of Health and Human Services, the Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority.
As of July 20, 2023, about 95 million influenza vaccines were being distributed in the U.S. The U.S. CDC recommends an annual flu shot for most people, which is offered at most health clinics and community pharmacies in the U.S.

The U.S. Centers for Disease Control and Prevention (CDC) today announced an Emergency Preparedness and Response COCA Call on July 20, 2023, where participants will learn how to prevent, diagnose, and treat malaria.
And how the biology of the pathogen contributes to the clinical management of the disease,
And how CDC and state and local health departments respond to the locally acquired mosquito-borne malaria cases in Florida and Texas.
Previously, the CDC issued a Health Alert Network Health Advisory on June 26, 2023, to share information on the recent identification of locally acquired, mosquito-transmitted malaria cases (P. vivax) in Florida and Texas.
Although these are the first documented instances of locally transmitted malaria in the U.S. since 2003, approximately 2,000 malaria cases are diagnosed and treated in the U.S. each year.
Malaria cases are primarily confirmed in individuals returning from travel to malaria-endemic countries such as Brazil and Cuba.
These malaria infections present a potential risk of subsequent transmission domestically since Anopheles mosquitoes, capable of transmitting malaria, are broadly distributed across the United States.
For example, locally-infected and travel-related malaria cases in Florida were confirmed in July 2023.
During this COCA call on July 20, 2023, at 2:00 PM – 3:00 PM ET, at Webinar Link: https://www.zoomgov.com/j/1618112526, will learn CDC and state and local health departments are responding to the locally acquired mosquito-borne malaria cases in the U.S.
Unfortunately, the U.S. government has not approved a malaria-prevention vaccine as of July 18, 2023.

Pfizer Inc. and Flagship Pioneering, Inc. today announced a partnership to create a new pipeline of innovative medicines. The focus will be addressing unmet needs within Pfizer's core strategic areas of interest, including in broad patient populations and diseases with high potential to benefit from diverse technology platforms and modalities.
Pfizer will fund and have options to acquire development programs.
Under the terms of the novel agreement, Flagship and Pfizer will each invest $50M upfront to explore opportunities to develop ten single-asset programs by leveraging Flagship's ecosystem of more than 40 human health companies and multiple biotechnology platforms.
To date, Flagship has deployed over $3.4 billion in capital toward the founding and growth of its pioneering companies alongside more than $26 billion of follow-on investments from other institutions.
Per the new agreement, Flagship and its bioplatform companies can receive up to $700M in milestones and royalties for each successfully commercialized program.
"At Pfizer, we are expanding our efforts to pursue potential breakthrough science with unique approaches and funding mechanisms designed to leverage the dynamic scientific ecosystem," said Mikael Dolsten, M.D., Ph.D., Chief Scientific Officer and President, Worldwide Research, Development and Medical of Pfizer, in a press release on July 18, 2023.
"This collaboration is an exciting opportunity for Pfizer to bring deep scientific expertise and apply our development and regulatory strength to Flagship's diverse portfolio of technology platforms, translating early-stage innovation to potential medicines."

Vaxxinity, Inc. today announced new data from an early-stage clinical trial demonstrating that antibodies derived from its investigational immunotherapeutic for Parkinson's disease, UB-312, slows the seeding of alpha-synuclein (aSyn) in cerebrospinal fluid (CSF) of patients as demonstrated using multiple target engagement assays.
These data signify that UB-312 has established clear target engagement in Parkinson's disease (PD) patient CSF.
Jean-Cosme Dodart, Ph.D., SVP of Research at Vaxxinity, commented in an email, “The more data we see from our UB-312 program, the more excited I get for the future of this vaccine and its potential positive impacts for patients with PD or other a-synucleopathies."
"Demonstrating target engagement in PD patients immunized with UB-312 is an exciting milestone which encourages us to push this program further into clinical development.”
UB-312 is a UBITh®-enhanced synthetic peptide-based vaccine designed to target aggregated forms of aSyn, the toxic species that underlies PD and other synucleinopathies.
"Our candidate has shown target engagement of the toxic species of alpha-synuclein in patients, demonstrating not only proof of our technology platform but also proof of the mechanism of our vaccine-derived antibodies specifically engaging with the toxic target in vivo," said Mei Mei Hu, CEO of Vaxxinity, in a press release on July 17, 2023.
"Showing target engagement has always been a key challenge to overcome in neurodegeneration and is of critical importance when demonstrated – a milestone worth celebrating."
"It is beyond our expectation to see this in our Phase 1 clinical trial."
"We are endlessly grateful to the patients who participated and to The Michael J. Fox Foundation and our collaborators for their work on these cutting-edge assays that supported this breakthrough."
Last month, Vaxxinity announced clinical data from Part B of its Phase 1 clinical trial of UB-312 demonstrating that UB-312 was well-tolerated and induced anti-aSyn antibody responses in participants with early PD and that antibodies were detectable in the CSF.
As part of this trial, The Michael J. Fox Foundation (MJFF) funded a 2-year collaborative project between Vaxxinity, the Mayo Clinic, and UTHealth Houston to analyze CSF collected from patients and to conduct exploratory research to characterize the anti-aSyn antibodies produced after UB-312 administration and assess target engagement.
Mark Frasier, Ph.D., Chief Scientific Officer of MJFF, commented, "Integration of critical biomarker insight into therapeutic development programs is essential for building confidence in the treatment approach and designing informative trials. We're pleased to support efforts of this kind that can have a major impact for people with Parkinson's disease."