Search API
CSL Seqirus today announced it was selected by the Biomedical Advanced Research and Development Authority (BARDA) to deliver one bulk lot of H5N8 A/Astrakhan antigen to the U.S. government.
This acquisition of a bulk lot will increase BARDA's stockpile of vaccines to support rapid response in an associated influenza pandemic.
CSL Seqirus has been working with BARDA in a longstanding partnership for over a decade, including numerous R&D and manufacturing activities and awards supporting BARDA's pandemic preparedness objectives.
Confirmed on August 28, 2023, this is the third award CSL Seqirus has received from BARDA in the last two years related to the ongoing outbreak of HPAI in the United States.
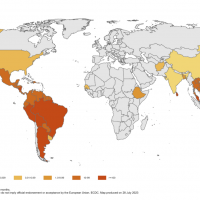
As of August 2023, the Pan American Health Organization reported H5N1 viruses (clade 2.3.4.4b) had been detected in 15 countries in Latin America, the Caribbean, the United States, and Canada over the past 18 months.
This award follows the February 2022 award to produce an H5N8 A/Astrakhan virus vaccine seed and the subsequent October 2022 announcement of the selection of CSL Seqirus to deliver an H5N8 A/Astrakhan virus vaccine candidate for assessment in a Phase 2 clinical study.
While the likelihood of sustained human-to-human transmission of bird flu is "low," according to the U.S. Centers for Disease Control and Prevention (CDC) and World Health Organization, there have been reported a small number of human cases of avian influenza A(H5), including one in the U.S. in April 2022, a case in Ecuador in January 20235 and Chile in March 2023.
"While human cases are rare, sporadic, and isolated, consistent detection of bird and mammalian cases demands vigilance," commented Marc Lacey, Executive Director, Pandemic Response Solutions, CSL Seqirus, in a press release.
"Ongoing surveillance and preparedness efforts are critical to minimize the public health risk."
CSL Seqirus used its cell-based influenza vaccine technology, as utilized for FDA-approved AUDENZ™ (Influenza A(H5N1) Monovalent Vaccine, Adjuvanted), to manufacture the H5N8 A/Astrakhan bulk vaccine at the company's Holly Springs, North Carolina, facility, which was built in partnership with BARDA.
CSL Seqirus has established and will maintain the required pandemic readiness to deliver 150 million doses of cell-based pandemic influenza vaccine within six months of an influenza pandemic declaration in the U.S.
This $46.3 million project has been supported in whole or in part with federal funds from the Department of Health and Human Services, Administration for Strategic Preparedness and Response; BARDA, under contract number 75A50122D00004.

According to media reports, the Dengue outbreak in Bangladesh accelerated in August 2023.
The Dhaka Tribune reported on August 26, 2023, that Bangladesh had its worst day ever for Dengue, with nine deaths and 1,960 hospitalized in a day.
This news increases Bangladesh's total number of dengue-related deaths to 537 in 2023.
Dengue is endemic in Bangladesh, resulting in high morbidity and mortality.
The World Health Organization says Dengue is the fastest-spreading mosquito-borne infectious disease and has emerged as a global public health problem.
As of August 28, 2023, two approved and various Dengue vaccine candidates are in development.
In addition to the ongoing Dengue outbreak, the U.S. CDC suggests various travel vaccines before visiting Bangladesh in 2023.
Pfizer Inc. recently announced in a press release that the European Commission (EC) has granted marketing authorization for ABRYSVO™, the company's bivalent respiratory syncytial virus (RSV) prefusion F (RSVpreF) vaccine, to help protect both infants through maternal immunization and older adults.
ABRYSVO is the first licensed vaccine designed and studied explicitly for maternal immunization. Now, a single dose of the vaccine could be administered in the EU between weeks 24 and 36 of gestation.
In addition, ABRYSVO has been studied in adults 60 and older.
"The approval of ABRYSVO in Europe marks significant progress in the scientific community's efforts to provide meaningful protection against RSV, a common respiratory virus that could potentially be severe and even life-threatening, especially for infants and older adults," commented Annaliesa Anderson, Ph.D., Senior Vice President and Head Vaccine Research and Development, Pfizer on August 24, 2023.
"Last year's significant number of newborns, children, and adults being hospitalized across Europe demonstrated the immense need for protection against severe RSV cases. The approval of the vaccine for both older adults and infants through maternal immunization is a triumph for public health, and we hope to see a tremendous impact for future (RSV) seasons."
This authorization is valid in all 27 EU member states, plus Iceland, Liechtenstein, and Norway.
RSV is a contagious virus and a common cause of respiratory illness worldwide.
In the EU, approximately 245,000 yearly hospital admissions were associated with RSV in children younger than five.
The disease burden for older adults is also significant. Each year, the virus causes more than 270,000 hospitalizations and about 20,000 deaths in individuals and older.
The virus can affect an infected individual's lungs and breathing passages, potentially causing severe illness or death.
As of August 26, 2023, two RSV vaccines are approved for seniors and available at clinics and pharmacies in the United States.

The U.S. Centers for Disease Control and Prevention (CDC) published a Morbidity and Mortality Weekly Report (MMWR) on August 25, 2023, with updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the 2023–24 Influenza Season.
The primary updates to this new MMWR include the following two topics: 1) the composition of 2023–24 U.S. seasonal influenza vaccines and 2) updated recommendations regarding influenza vaccination of persons with egg allergy.
First, the composition of 2023–24 U.S. influenza vaccines includes an update to the influenza A(H1N1)pdm09 component. U.S.-licensed influenza vaccines will contain HA derived from:
1) an influenza A/Victoria/4897/2022 (H1N1)pdm09-like virus (for egg-based vaccines) or an influenza A/Wisconsin/67/2022 (H1N1)pdm09-like virus (for cell culture-based and recombinant vaccines).
2) an influenza A/Darwin/9/2021 (H3N2)-like virus (for egg-based vaccines) or an influenza A/Darwin/6/2021 (H3N2)-like virus (for cell culture-based and recombinant vaccines).
3) an influenza B/Austria/1359417/2021 (Victoria lineage)-like virus.
4) an influenza B/Phuket/3073/2013 (Yamagata lineage)-like virus.
Second, the ACIP recommends that all persons aged ≥6 months with egg allergy should receive an influenza vaccine.
Any influenza vaccine (egg-based or non-egg-based) that is otherwise appropriate for the recipient’s age and health status can be used.
It is no longer recommended that persons with allergic reactions to eggs involving symptoms other than urticaria should be vaccinated in an inpatient or outpatient medical setting supervised by a healthcare provider who can recognize and manage severe allergic reactions if an egg-based vaccine is used.
Egg allergy alone necessitates no additional safety measures for influenza vaccination beyond those recommended for any vaccine recipient, regardless of the severity of a previous reaction to an egg.
Furthermore, all flu shots for 2023-2024 should be administered in settings in which personnel and equipment needed for rapid recognition and treatment of acute hypersensitivity reactions are available.
A summary of these recommendations is posted on this CDC webpage.

The U.S. Administration for Strategic Preparedness and Response's Biomedical Advanced Research and Development Authority (BARDA) today awarded $10 million to Johnson & Johnson Innovation for a competition through project Blue Knight™.
"As the virus continues to evolve, we need new tools that keep pace with those changes," said Assistant Secretary for Preparedness and Response Dawn O'Connell in a press release on August 22, 2023.
This BARDA award is in alignment with 'Project NextGen' which focuses on advancing solutions aimed at addressing health security threats and improving preparedness,
Announced in May 2023, the U.S. Department of Health and Human Services Project NextGen is a $5 billion initiative led by BARDA in partnership with the National Institute of Allergy and Infectious Diseases, coordinates activities across the federal government and the private sector to advance innovative vaccines and therapeutics into clinical trials, regulatory review, and potential commercial availability for the American people.
Announced on May 11, 2023, the Blue Knight challenge offers current and alumni Blue Knight residents and their collaborators the opportunity to apply for the chance to receive funding to help them reach their critical developmental milestones.
Learn more about Blue Knight and hear from current companies at this Johnson & Johnson Innovation LLC link.

Regeneron Pharmaceuticals, Inc. today announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA) entered into an agreement with the Company to support the clinical development, clinical manufacturing, and regulatory licensure process of a next-generation COVID-19 monoclonal antibody (mAb) therapy for the prevention of SARS-CoV-2 infections, which cause COVID-19 in people.
The agreement is part of the U.S. Department of Health and Human Services (HHS) 'Project NextGen' initiative to advance innovative vaccines and therapeutics for COVID-19.
Regeneron's most advanced next-generation antibody candidate under this agreement is expected to enter clinical trials in 2023.
For the new COVID-19 program announced on August 22, 2023, HHS will fund up to 70% of Regeneron's costs for certain clinical development activities for a next-generation mAb therapy.
The new BARDA contract has an estimated value of up to approximately $326 million of government funding.
Regeneron's first COVID-19 mAb cocktail, REGEN-COV, was granted Emergency Use Authorization in November 2020, with nearly 3 million doses delivered to the U.S. Government between 2020 and 2022.
"We're pleased to expand our longstanding BARDA relationship, which is predicated on Regeneron's decades of investment in deep scientific research and enabling technologies," said Leonard S. Schleifer, M.D., Ph.D., Board Co-Chair, President and Chief Executive Officer of Regeneron, in a press release.
"Although COVID-19 has moved to an endemic stage, many people – including those with immunocompromising conditions – continue to face exposure that impacts their everyday life and could cause serious health consequences."
Previously, the U.S. CDC wrote some immunocompromised people benefit from mAb therapy instead of COVID-19 vaccination.
Under the NextGen project structure, Regeneron independently invents and proposes an antibody candidate, which BARDA and Regeneron will evaluate and agree upon for further development, manufacturing, and regulatory activities.
BARDA and Regeneron have previously worked together to deliver novel medicines for Ebola.
The new program announced today falls under Regeneron and BARDA's ongoing Other Transactions Agreement initiated in 2017 to develop a portfolio of antibodies targeting up to ten pathogens that pose significant risks to public health.

Novavax, Inc. today announced that its updated protein-based XBB COVID vaccine candidate induced neutralizing antibody responses to the EG.5.1 and XBB.1.16.6 subvariants in small pre-clinical studies.
As of August 22, 2023, SARS-CoV-2 coronavirus XBB sublineage variants are overwhelmingly responsible for the majority of current COVID-19 cases in the U.S. and European Union.
"Our data have shown that Novavax's protein-based COVID vaccine induces broadly neutralizing responses against XBB subvariants, including EG.5.1 and XBB.1.16.6," commented Filip Dubovsky, President of Research and Development, Novavax, in a press release.
Non-clinical data previously showed that Novavax's COVID vaccine candidate induced functional immune responses for XBB.1.5, XBB.1.16, and XBB.2.3 variants, indicating a broad response that could potentially be applicable for forward-drift variants.
Novavax is submitting applications for its XBB.1.5 COVID vaccine candidate to regulatory authorities globally.
Novavax COVID-19 vaccine brands (Nuvaxovid, CovoVax, NVX-CoV2373, TAK-019) have been authorized in about 40 markets.
The Novavax COVID-19 Vaccine, Adjuvanted, has not been approved or licensed by the U.S. FDA but is authorized for emergency use. Novavax vaccines are available in specific clinics and pharmacies in the U.S.

CARsgen Therapeutics Holdings Limited today announced a collaboration agreement with Moderna Inc. to investigate CARsgen's investigational Claudin18.2 CAR T-cell product candidate (CT041) in combination with Moderna's investigational Claudin18.2 mRNA cancer vaccine.
CT041 (satricabtagene autoleucel) is CARsgen's autologous CAR T-cell product being investigated for potentially treating gastric, pancreatic, and other specified digestive system cancers.
It is currently in multiple ongoing clinical studies in China and North America.
"CT041 is the most advanced solid tumor CAR-T in development (pivotal phase II) and continues to show promise in treating gastric and pancreatic cancers. In our quest to make cancer curable, we are continuously exploring multiple modalities to eradicate tumors. Attacking tumors with CAR T-cell therapy in combination with a cancer vaccine could potentially provide greater clinical benefit to patients." said Dr. Zonghai Li, Founder, Chairman of the Board, Chief Executive Officer, and Chief Scientific Officer of CARsgen Therapeutics Holdings Limited, in a press release on August 21, 2023.
Dr. Li added, "Moderna has clearly established itself as a scientific and commercial leader in mRNA-based vaccines and therapeutics, and we are pleased to partner with Moderna to explore a potential synergism between our innovative therapies."
Moderna is developing an investigational off-the-shelf mRNA cancer vaccine that encodes for the Claudin18.2 protein, a tumor-associated antigen.
The collaboration contemplates conducting preclinical studies and a phase I clinical trial to evaluate CT041 in combination with Moderna's Claudin18.2 mRNA cancer vaccine.
"We are pleased to partner with CARsgen to explore the potential synergy of CAR-T with an investigational mRNA cancer vaccine that encodes for the Claudin18.2 protein. Claudin18.2 is a promising therapeutic target to potentially treat multiple cancer types with high unmet medical need. We continue to deliver on the promise of mRNA science to create a new generation of transformative medicines in oncology," added Dr. Lin Guey, Chief Scientific Officer of External Research Ventures, Moderna.
CARsgen is a biopharmaceutical company with operations in China, and the U.S. focused on innovative CAR T-cell therapies for treating hematologic malignancies and solid tumors.